Nomograms for predicting the overall and cancer-specific survival in patients with non-urothelial carcinoma of the bladder: A population-based study
DOI:
https://doi.org/10.17305/bb.2023.9881Keywords:
Non-urothelial, bladder cancer, nomogram, Surveillance, Epidemiology, and End Results (SEER)Abstract
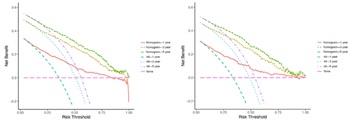
Non-urothelial carcinoma of the bladder (NUCB) is a relatively rare condition, with limited comprehensive studies conducted to date. This research aims to establish nomograms for forecasting overall survival (OS) and cancer-specific survival (CSS) in NUCB patients. It utilizes data of 2,522 patients from the Surveillance, Epidemiology, and End Results (SEER) database spanning from 2004 to 2015. The effectiveness of these nomograms was assessed using receiver operating characteristic (ROC) curves, calibration curves and decision curve analysis (DCA). Key independent predictors for OS included age, race, marital status, histological variants, grade, T stage, N stage, M stage, radical cystectomy and chemotherapy administration. For CSS, the predictors were similar, encompassing age, sex, marital status, histological variants, grade, T stage, N stage, M stage, radical cystectomy and chemotherapy. The nomograms showed strong predictive accuracy. In the training cohort, the area under the curve (AUC) values were 0.796 (OS) and 0.799 (CSS) at 1-year, 0.807 (OS) and 0.824 (CSS) at 3-year, and 0.807 (OS) and 0.827 (CSS) at 5-year intervals. In the validation cohort, AUC values were 0.798 (OS) and 0.798 (CSS) at 1-year, 0.810 (OS) and 0.826 (CSS) at 3-year, and 0.811 (OS) and 0.825 (CSS) at 5-year intervals, consistently around 0.8. Calibration curves indicated high congruence between predicted and actual probabilities of OS and CSS, while DCA demonstrated the models' substantial clinical utility. Overall, this study successfully developed and validated prognostic nomograms for NUCB, capable of accurately predicting OS and CSS at 1-, 3-, and 5-years, thereby offering valuable support in clinical decision-making and the design of clinical trials.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2023 Zhenchi Li, Jiangping Wang, Zhibin Xu, Mei Wang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2023-11-16
Published 2024-05-02









