Procalcitonin and C-reactive protein-based decision tree model for distinguishing PFAPA flares from acute infections
DOI:
https://doi.org/10.17305/bjbms.2016.974Keywords:
periodic fever, PCT, CRP, bacterial infection, diagnosisAbstract
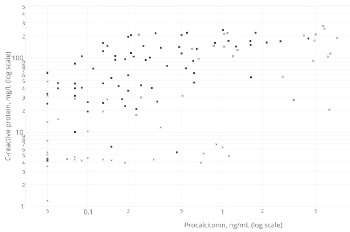
As no specific laboratory test has been identified, PFAPA (periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis) remains a diagnosis of exclusion. We searched for a practical use of procalcitonin (PCT) and C-reactive protein (CRP) in distinguishing PFAPA attacks from acute bacterial and viral infections. Levels of PCT and CRP were measured in 38 patients with PFAPA and 81 children diagnosed with an acute bacterial (n=42) or viral (n=39) infection. Statistical analysis with the use of the C4.5 algorithm resulted in the following decision tree: viral infection if CRP≤19.1 mg/L; otherwise for cases with CRP>19.1 mg/L: bacterial infection if PCT>0.65ng/mL, PFAPA if PCT≤0.65 ng/mL. The model was tested using a 10-fold cross validation and in an independent test cohort (n=30), the rule’s overall accuracy was 76.4% and 90% respectively. Although limited by a small sample size, the obtained decision tree might present a potential diagnostic tool for distinguishing PFAPA flares from acute infections when interpreted cautiously and with reference to the clinical context.
Citations
Downloads
References
Thomas KT, Feder HM Jr, Lawton AR, Edwards KM. Periodic fever syndrome in children. J Pediatr 1999; 135(1): 15–21. http://dx.doi.org/10.1016/S0022-3476(99)70321-5
Gioia SA, Bedoni N, von Scheven-Gête A, Venoni F, Superti-Furga A, Hofer M, et al. Analysis of the genetic basis of periodic fever with aphthous stomatitis, pharyngitis, and cervical adenitis (PFAPA) syndrome. Sci Rep 2015; 19; 5:10200.
doi: 10.1038/srep10200
Feder HM, Salazar JC. A clinical review of 105 patients with PFAPA (a periodic fever syndrome). Acta Paediatr 2010; 99(2):178-184. doi: 10.1111/j.1651-2227.2009.01554.x
Kyvsgaard N, Mikkelsen T, Korsholm J, Veirum JE, Herlin T. Periodic fever associated with aphthous stomatitis, pharyngitis and cervical adenitis. Dan Med J 2012; 59(7):A4452.
Førsvoll J, Oymar K. C-reactive protein in the periodic fever, aphthous stomatitis, pharyngitis and cervical adenitis (PFAPA) syndrome. Acta Paediatr 2007; 96(11):1670-1673.
http://dx.doi.org/10.1111/j.1651-2227.2007.00499.x
Yoshihara T, Imamura T, Yokoi K, Shibata M, Kano G, Osone S, et al. Potential use of procalcitonin concentrations as a diagnostic marker of the PFAPA syndrome. Eur J Pediatr 2007; 166(6):621-622. http://dx.doi.org/10.1007/s00431-006-0281-2
Yazgan H, Keleş E, Yazgan Z, Gebeşçe A, Demirdöven M. C-reactive protein and procalcitonin during febrile attacks in PFAPA syndrome. Int J Pediatr Otorhinolaryngol 2012; 76(8):1145-1147. http://dx.doi.org/10.1016/j.ijporl.2012.04.022
Førsvoll J, Kristoffersen EK, Øymar K. Is there a role for procalcitonin in the evaluation of children with PFAPA syndrome? Ann Paediatr Rheum 2012; 1(3):171-175.
http://dx.doi.org/10.5455/apr.092520121447
Brown KL, Wekell P, Osla V, Sundqvist M, Sävman K, Fasth A, et al. Profile of blood cells and inflammatory mediators in periodic fever, aphthous stomatitis, pharyngitis and adenitis (PFAPA) syndrome. BMC Pediatr 2010; 10:65.
http://dx.doi.org/10.1186/1471-2431-10-65
Kraszewska-Głomba B, Matkowska-Kocjan A, Szenborn L. The pathogenesis of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome: a review of current research. Mediators Inflamm 2015; 2015:563876.
http://dx.doi.org/10.1155/2015/563876
Lee H. Procalcitonin as a biomarker of infectious diseases. Korean J Intern Med 2013; 28:285-291.
http://dx.doi.org/10.3904/kjim.2013.28.3.285
Assicot M, Gendrel D, Carsin H, Raymond J, Guilbaud J, Bohuon C. High serum procalcitonin concentrations in patients with sepsis and infection. Lancet 1993; 341(8844):515-518. http://dx.doi.org/10.1016/0140-6736(93)90277-N
Simon L, Saint-Louis P, Amre DK, Lacroix J, Gauvin F. Procalcitonin and C-reactive protein as markers of bacterial infection in critically ill children at onset of systemic inflammatory response syndrome. Pediatr Crit Care Med. 2008; 9(4):407-413.
http://dx.doi.org/10.1097/PCC.0b013e31817285a6
Thermo Scientific [Internet]. Sepsis Marker PCT; c2013 [cited January 2016]. Available from: http://www.procalcitonin.com/default.aspx?tree=_2_2
Quinlan, J. R. C4.5: Programs for machine learning. 1st ed. San Francisco, CA: Morgan Kaufmann Publishers; 1993.
Sundqvist M, Wekell P, Osla V, Bylund J, Christenson K, Sävman K, et al. Increased intracellular oxygen radical production in neutrophils during febrile episodes of periodic fever, aphthous stomatitis, pharyngitis, and cervical adenitis syndrome. Arthritis Reum 2013; 65(11):2971-2983.
http://dx.doi.org/10.1002/art.38134
Førsvoll J, Kristoffersen EK, Oymar K. Elevated levels of CXCL10 in the Periodic Fever, Aphthous stomatitis, Pharyngitis and cervical Adenitis syndrome (PFAPA) during and between febrile episodes; an indication of a persistent activation of the innate immune system. Pediatr Rheumatol Online J 2013; 11(1):38. http://dx.doi.org/10.1186/1546-0096-11-38
Stojanov S, Lapidus S, Chitkara P, Feder H, Salazar JC, Fleisher TA, et al. Periodic fever, aphthous stomatitis, pharyngitis, and adenitis (PFAPA) is a disorder of innate immunity and Th1 activation responsive to IL-1 blockade. Proc Natl Acad Sci U S A 2011; 108:7148–7153.
http://dx.doi.org/10.1073/pnas.1103681108
Kolly L, Busso N, von Scheven-Gete A, Bagnoud N, Moix I, Holzinger D, et al. Periodic fever, aphthous stomatitis, pharyngitis, cervical adenitis syndrome is linked to dysregulated monocyte IL-1beta production. J Allergy Clin Immunol 2013; 131:1635–1643.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-02-10
Published 2016-03-10









