Tumor suppressor maspin as a modulator of host immune response to cancer
DOI:
https://doi.org/10.17305/bjbms.2015.783Keywords:
Anticancer immunity, immunosuppression, immunogenicity, cancer immunoediting, innate immunity, adaptive immunity, serine protease inhibitors, tumor suppressor, maspin, autoantigen, differentiation, epigenetic, cancer stem cells, cellular plasticity, tumorAbstract
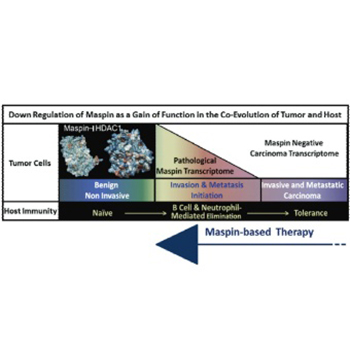
Despite the promising clinical outcome, the primary challenge of the curative cancer immunotherapy is to overcome the dichotomy of the immune response: tumor-evoked immunostimulatory versus tumor-induced immunosuppressive. The goal needs to be two-fold, to re-establish sustainable antitumor-cancer immunity and to eliminate immunosuppression. The successful elimination of cancer cells by immunosurveillance requires the antigenic presentation of the tumor cells or tumor-associated antigens and the expression of immunostimulatory cytokines and chemokines by cancer and immune cells. Tumors are heterogeneous and as such, some of the tumor cells are thought to have stem cell characteristics that enable them to suppress or desensitize the host immunity due to acquired epigenetic changes. A central mechanism underlying tumor epigenetic instability is the increased histone deacetylase (HDAC)-mediated repression of HDAC-target genes regulating homeostasis and differentiation. It was noted that pharmacological HDAC inhibitors are not effective in eliminating tumor cells partly because they may induce immunosuppression. We have shown that epithelial-specific tumor suppressor maspin, an ovalbumin-like non-inhibitory serine protease inhibitor, reprograms tumor cells toward better differentiated phenotypes by inhibiting HDAC1. Recently, we uncovered a novel function of maspin in directing host immunity towards tumor elimination. In this review, we discuss the maspin and maspin/HDAC1 interplay in tumor biology and immunology. We propose that maspin based therapies may eradicate cancer.
Citations
Downloads
References
Schreiber RD, Old LJ, Smyth MJ. Cancer immunoediting: Integrating immunity's roles in cancer suppression and promotion. Science 2011;331(6024):1565-70. http://dx.doi.org/10.1126/science.1203486.
Dunn GP, Old LJ, Schreiber RD. The three Es of cancer immunoediting. Annu Rev Immunol 2004;22:329-60. http://dx.doi.org/10.1146/annurev.immunol.22.012703.104803.
Mittal D, Gubin MM, Schreiber RD, Smyth MJ. New insights into cancer immunoediting and its three component phases – Elimination, equilibrium and escape. Curr Opin Immunol 2014;27:16-25. http://dx.doi.org/10.1016/j.coi.2014.01.004.
Tu E, Chia PZ, Chen W. TGFß in T cell biology and tumor immunity: Angel or devil? Cytokine Growth Factor Rev 2014;25(4):423-35. http://dx.doi.org/10.1016/j.cytogfr.2014.07.014.
Fridlender ZG, Sun J, Kim S, Kapoor V, Cheng G, Ling L, et al. Polarization of tumor-associated neutrophil phenotype by TGF-beta: "N1" versus "N2" TAN. Cancer Cell 2009;16(3):183-94. http://dx.doi.org/10.1016/j.ccr.2009.06.017.
De Smet C, Lurquin C, De Plaen E, Brasseur F, Zarour H, De Backer O, et al. Genes coding for melanoma antigens recognised by cytolytic T lymphocytes. Eye (Lond) 1997;11:243-8. http://dx.doi.org/10.1038/eye.1997.59.
Law RH, Zhang Q, McGowan S, Buckle AM, Silverman GA, Wong W, et al. An overview of the serpin superfamily. Genome Biol 2006;7(5):216. http://dx.doi.org/10.1186/gb-2006-7-5-216.
Gatto M, Iaccarino L, Ghirardello A, Bassi N, Pontisso P, Punzi L, et al. Serpins, immunity and autoimmunity: Old molecules, new functions. Clin Rev Allergy Immunol 2013;45(2):267-80. http://dx.doi.org/10.1007/s12016-013-8353-3.
Li Y, Wang LX, Pang P, Twitty C, Fox BA, Aung S, et al. Cross-presentation of tumor associated antigens through tumor-derived autophagosomes. Autophagy 2009;5(4):576-7. http://dx.doi.org/10.4161/auto.5.4.8366.
Vidalino L, Doria A, Quarta SM, Crescenzi M, Ruvoletto M, Frezzato F, et al. SERPINB3 expression on B-cell surface in autoimmune diseases and hepatitis C virus-related chronic liver infection. Exp Biol Med (Maywood) 2012;237(7):793-802. http://dx.doi.org/10.1258/ebm.2012.012024.
El-Rachkidy RG, Young HS, Griffiths CE, Camp RD. Humoral autoimmune responses to the squamous cell carcinoma antigen protein family in psoriasis. J Invest Dermatol 2008;128(9):2219-24. http://dx.doi.org/10.1038/jid.2008.71.
Chokshi NY, Sicherer SH. Molecular diagnosis of egg allergy: An update. Expert Rev Mol Diagn 2015;15(7):895-906. http://dx.doi.org/10.1586/14737159.2015.1041927.
Caubet JC, Kondo Y, Urisu A, Nowak-Wegrzyn A. Molecular diagnosis of egg allergy. Curr Opin Allergy Clin Immunol 2011;11(3):210-5. http://dx.doi.org/10.1097/ACI.0b013e3283464d1b.
Scambia G, Benedetti Panici P, Foti E, Amoroso M, Salerno G, Ferrandina G, et al. Squamous cell carcinoma antigen: Prognostic significance and role in the monitoring of neoadjuvant chemotherapy response in cervical cancer. J Clin Oncol 1994;12(11):2309-16.
Turato C, Simonato D, Quarta S, Gatta A, Pontisso P. MicroRNAs and SerpinB3 in hepatocellular carcinoma. Life Sci 2014;100(1):9-17. http://dx.doi.org/10.1016/j.lfs.2014.01.073.
Cacoub P, Frémeaux-Bacchi V, De Lacroix I, Guillien F, Kahn MF, Kazatchkine MD, et al. A new type of acquired C1 inhibitor deficiency associated with systemic lupus erythematosus. Arthritis Rheum 2001;44(8):1836-40. http://dx.doi.org/10.1002/1529-0131(200108)44:8<1836::AID-ART321>3.0.CO;2-Y.
Frazer JK, Jackson DG, Gaillard JP, Lutter M, Liu YJ, Banchereau J, et al. Identification of centerin: A novel human germinal center B cell-restricted serpin. Eur J Immunol 2000;30(10):3039-48. http://dx.doi.org/10.1002/1521-4141(200010)30:10<3039::AID-IMMU3039>3.0.CO;2-H.
Dzinic SH, Chen K, Thakur A, Kaplun A, Bonfil RD, Li X, et al. Maspin expression in prostate tumor elicits host anti-tumor immunity. Oncotarget 2014;5(22):11225-36. http://dx.doi.org/10.18632/oncotarget.2615.
Li X, Yin S, Meng Y, Sakr W, Sheng S. Endogenous inhibition of histone deacetylase 1 by tumor-suppressive maspin. Cancer Res 2006;66(18):9323-9. http://dx.doi.org/10.1158/0008-5472.CAN-06-1578.
Li X, Kaplun A, Lonardo F, Heath E, Sarkar FH, Irish J, et al. HDAC1 inhibition by maspin abrogates epigenetic silencing of glutathione S-transferase pi in prostate carcinoma cells. Mol Cancer Res 2011;9(6):733-45. http://dx.doi.org/10.1158/1541-7786.MCR-10-0505.
Bodenstine TM, Seftor RE, Khalkhali-Ellis Z, Seftor EA, Pemberton PA, Hendrix MJ. Maspin: Molecular mechanisms and therapeutic implications. Cancer Metastasis Rev 2012;31(3-4):529-51. http://dx.doi.org/10.1007/s10555-012-9361-0.
Cher ML, Biliran HR Jr, Bhagat S, Meng Y, Che M, Lockett J, et al. Maspin expression inhibits osteolysis, tumor growth, and angiogenesis in a model of prostate cancer bone metastasis. Proc Natl Acad Sci U S A 2003;100(13):7847-52. http://dx.doi.org/10.1073/pnas.1331360100.
Luo JL, Tan W, Ricono JM, Korchynskyi O, Zhang M, Gonias SL, et al. Nuclear cytokine-activated IKKalpha controls prostate cancer metastasis by repressing Maspin. Nature 2007;446(7136):690-4. http://dx.doi.org/10.1038/nature05656.
Li X, Chen D, Yin S, Meng Y, Yang H, Landis-Piwowar KR, et al. Maspin augments proteasome inhibitor-induced apoptosis in prostate cancer cells. J Cell Physiol 2007;212(2):298-306. http://dx.doi.org/10.1002/jcp.21102.
Jiang N, Meng Y, Zhang S, Mensah-Osman E, Sheng S. Maspin sensitizes breast carcinoma cells to induced apoptosis. Oncogene 2002;21(26):4089-98. http://dx.doi.org/10.1038/sj.onc.1205507.
Bernardo MM, Kaplun A, Dzinic SH, Li X, Irish J, Mujagic A, et al. Maspin expression in prostate tumor cells averts stemness and stratifies drug sensitivity. Cancer Res 2015;75(18):3970-9. http://dx.doi.org/10.1158/0008-5472.CAN-15-0234.
Bernardo MM, Meng Y, Lockett J, Dyson G, Dombkowski A, Kaplun A, et al. Maspin reprograms the gene expression profile of prostate carcinoma cells for differentiation. Genes Cancer 2011;2(11):1009-22. http://dx.doi.org/10.1177/1947601912440170.
Ning X, Shu J, Du Y, Ben Q, Li Z. Therapeutic strategies targeting cancer stem cells. Cancer Biol Ther 2013;14(4):295-303. http://dx.doi.org/10.4161/cbt.23622.
Machtens S, Serth J, Bokemeyer C, Bathke W, Minssen A, Kollmannsberger C, et al. Expression of the p53 and Maspin protein in primary prostate cancer: Correlation with clinical features. Int J Cancer 2001;95(5):337-42. http://dx.doi.org/10.1002/1097-0215(20010920)95:5<337::AID-IJC1059>3.0.CO;2-1.
Pierson CR, McGowen R, Grignon D, Sakr W, Dey J, Sheng S. Maspin is up-regulated in premalignant prostate epithelia. Prostate 2002;53(4):255-62. http://dx.doi.org/10.1002/pros.10107.
Lonardo F, Guan H, Dzinic S, Sheng S. Maspin expression patterns differ in the invasive versus lepidic growth pattern of pulmonary adenocarcinoma. Histopathology 2014;65(6):757-63. http://dx.doi.org/10.1111/his.12485.
Laresgoiti-Servitje E. A leading role for the immune system in the pathophysiology of preeclampsia. J Leukoc Biol 2013;94(2):247-57. http://dx.doi.org/10.1189/jlb.1112603.
Qi YH, Teng F, , Zhou Q, Liu YX, Wu JF, et al. Unmethylated-maspin DNA in maternal plasma is associated with severe preeclampsia. Acta Obstet Gynecol Scand 2015;94(9):983-8. http://dx.doi.org/10.1111/aogs.12691.
Taglauer ES, Gundogan F, Johnson KL, Scherjon SA, Bianchi DW. Chorionic plate expression patterns of the maspin tumor suppressor protein in preeclamptic and egg donor placentas. Placenta 2013;34(4):385-7. http://dx.doi.org/10.1016/j.placenta.2013.01.008.
Das UN. Cytokines, angiogenic, and antiangiogenic factors and bioactive lipids in preeclampsia. Nutrition 2015;31(9):1083-95. http://dx.doi.org/10.1016/j.nut.2015.03.013.
Besgen P, Trommler P, Vollmer S, Prinz JC. Ezrin, maspin, peroxiredoxin 2, and heat shock protein 27: Potential targets of a streptococcal-induced autoimmune response in psoriasis. J Immunol 2010;184(9):5392-402. http://dx.doi.org/10.4049/jimmunol.0903520.
Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, et al. Corrigendum: B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol 2014;15(2):205. http://dx.doi.org/10.1038/ni0214-205a.
Palanichamy A, Bauer JW, Yalavarthi S, Meednu N, Barnard J, Owen T, et al. Neutrophil-mediated IFN activation in the bone marrow alters B cell development in human and murine systemic lupus erythematosus. J Immunol 2014;192(3):906-18. http://dx.doi.org/10.4049/jimmunol.1302112.
Biliran H Jr, Sheng S. Pleiotrophic inhibition of pericellular urokinase-type plasminogen activator system by endogenous tumor suppressive maspin. Cancer Res 2001;61(24):8676-82.
Kaplun A, Dzinic S, Bernardo M, Sheng S. Tumor suppressor maspin as a rheostat in HDAC regulation to achieve the fine-tuning of epithelial homeostasis. Crit Rev Eukaryot Gene Expr 2012;22(3):249-58. http://dx.doi.org/10.1615/CritRevEukarGeneExpr.v22.i3.80.
Kelly TK, De Carvalho DD, Jones PA. Epigenetic modifications as therapeutic targets. Nat Biotechnol 2010;28(10):1069-78. http://dx.doi.org/10.1038/nbt.1678.
Schotterl S, Brennenstuhl H, Naumann U. Modulation of immune responses by histone deacetylase inhibitors. Crit Rev Oncog 2015;20(1-2):139-54. http://dx.doi.org/10.1615/CritRevOncog.2014012393.
Cao K, Wang G, Li W, Zhang L, Wang R, Huang Y, et al. Histone deacetylase inhibitors prevent activation-induced cell death and promote anti-tumor immunity. Oncogene 2015 http://dx.doi.org/10.1038/onc.2015.46.
Kroesen M, Gielen P, Brok IC, Armandari I, Hoogerbrugge PM, Adema GJ. HDAC inhibitors and immunotherapy; a double edged sword? Oncotarget 2014;5(16):6558-72. http://dx.doi.org/10.18632/oncotarget.2289.
Kim ES, Lee JK. Histone deacetylase inhibitors decrease the antigen presenting activity of murine bone marrow derived dendritic cells. Cell Immunol 2010;262(1):52-7. http://dx.doi.org/10.1016/j.cellimm.2009.12.007.
Tao R, de Zoeten EF, Ozkaynak E, Chen C, Wang L, Porrett PM, et al. Deacetylase inhibition promotes the generation and function of regulatory T cells. Nat Med 2007;13(11):1299-307. http://dx.doi.org/10.1038/nm1652.
Wong DJ, Rao A, Avramis E, Matsunaga DR, Komatsubara KM, Atefi MS, et al. Exposure to a histone deacetylase inhibitor has detrimental effects on human lymphocyte viability and function. Cancer Immunol Res 2014;2(5):459-68. http://dx.doi.org/10.1158/2326-6066.CIR-13-0188.
Downloads
Additional Files
Published
How to Cite
Accepted 2015-10-08
Published 2015-10-25









