Evolution of the combined effect of different irrigation solutions and activation techniques on the removal of smear layer and dentin microhardness in oval-shaped root canal: An in-vitro study
DOI:
https://doi.org/10.17305/bjbms.2022.7440Keywords:
Root canal therapy, photon-induced photoacoustic streaming, passive ultrasonic irrigation, smear layer, dentin microhardnessAbstract
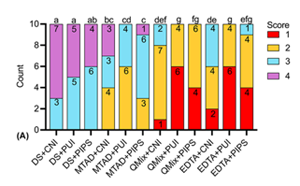
The aim of this in vitro study was to evaluate the effect of three final irrigants, namely QMix, MTAD and EDTA, combined with three irrigation techniques, namely conventional needle irrigation (CNI), passive ultrasonic irrigation (PUI) and photon-induced photoacoustic streaming (PIPS), on smear layer removal, dentin mineral content and microhardness in oval-shaped canals. 130 decoronated premolars with single, oval root canals were equally divided into1 blank control group and 12 treatment groups (n=10) according to the final irrigation protocols. Roots in treatment groups were instrumented with ProTaper Gold to size F4 and subjected to final irrigation. Smear layer removal was assessed by using a four-level scoring system under an environmental scanning electron microscope. Energy dispersive X-ray spectroscopy was performed to measure the dentin mineral content. Dentin microhardness was measured by Knoop microhardness testing. Statistical analysis of the data was performed by using Kruskal-Wallis test, followed by Dunn’s post hoc test with Bonferroni correction. PUI- and PIPS-activated QMix and EDTA removed smear layer more effectively than MTAD groups (p<0.05). Regarding the dentin mineral content and microhardness, QMix groups yielded the least calcium (Ca), phosphorus (P) and Ca/P ratio, followed by EDTA groups and MTAD groups (p<0.05). QMix groups produced significantly lower dentin microhardness values and higher hardness reduction percentages than MTAD groups (p<0.05). Within the limitations of the present study, it was concluded that QMix and EDTA were superior to MTAD in smear layer removal, especially when activated by PUI and PIPS, but these agents produced more pronounced effect on dentin mineral content and microhardness than MTAD.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Lu Shi, Jie Wan, Yunfei Yang, Ying Yao, Ruiming Yang, Wen Xie

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-06-27
Published 2023-01-06









