Inhibition of IRAK 1/4 alleviates colitis by inhibiting TLR4/ NF-κB pathway and protecting the intestinal barrier
DOI:
https://doi.org/10.17305/bjbms.2022.7348Keywords:
IRAK1/4, ulcerative colitis, TLR4/NF-κB, intestinal barrierAbstract
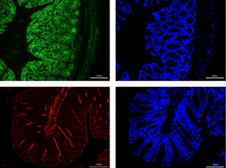
Interleukin-1 receptor-associated kinase 1/4 (IRAK1/4) is the main kinase of the Toll-like receptor (TLR)-mediated pathway, considered a new target for treating inflammatory diseases. Studies showed a significant correlation between TLRs and inflammatory responses in ulcerative colitis (UC). Therefore, in this study, after inducing experimental colitis in mice with 3% dextran sulfate sodium (DSS), different concentrations of IRAK1/4 inhibitors were administered intraperitoneally. Then, the disease activity index was assessed, including the degree of pathological damage, by HE staining. Subsequently, while western blotting detected the TLR4/NF-κB pathway and intestinal barrier protein expression (Zonula-1, Occludin, Claudin-1, JAM-A), real-time polymerase chain reaction (RT-PCR) detected the mRNA expression levels of IRAK1/4 and mucin1/2. Furthermore, the expression levels of Zonula-1 and occludin were detected by immunofluorescence, including the plasma FITC-dextran 4000 concentration, to evaluate intestinal barrier permeability. However, ELISA measured the expression of inflammatory factors to reflect intestinal inflammation in mice. Investigations showed that the IRAK 1/4 inhibitor significantly reduced clinical symptoms and pathological DSS-induced colitis damage in mice and then inhibited the cytoplasmic and nuclear translocation of NF-κB p65, including the phosphorylation of IκBα and reduction in downstream inflammatory factor production. Therefore, we established that the IRAK1/4 inhibitor effectively improves colitis induced by DSS, partly by inhibiting the TLR4/NF-κB pathway, reducing inflammation, and maintaining the integrity of the colonic barrier.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2022 Bo Yan, Xiangjie Li, Linxiang Zhou, Yuqing Qiao, Jing Wu, Lanlan Zha, Peilu Liu, Shuai Peng, Baixin Wu, Xiaoyun Yu, Lei Shen

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-06-06
Published 2022-10-23









