Serum microRNAs as biomarkers for the diagnosis of papillary thyroid carcinoma: a meta-analysis
DOI:
https://doi.org/10.17305/bjbms.2022.7343Keywords:
Circulating microRNAs, serum, diagnosis, meta-analysis, papillary thyroid carcinomaAbstract
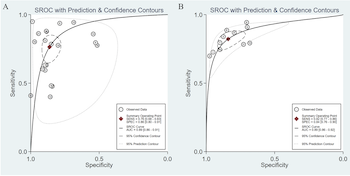
Papillary thyroid carcinoma (PTC) is the most common form of thyroid cancer. Several studies have proposed serum microRNAs (miRNAs) as novel biomarkers for diagnosing PTC. In this study, we conducted a meta-analysis aiming to investigate the overall diagnostic accuracy of serum miRNAs in PTC detection. Three online databases including PubMed, EMBASE and Cochrane Library were searched up to 1 May 2021. We systematically reviewed studies evaluating the value of serum miRNAs in diagnosing PTC, and then summarized the area under receiver operating characteristics curve (AUROC), sensitivity, specificity, and diagnostic odds ratio to assess the accuracy of serum miRNAs for the discrimination between patients with PTC and patients with benign thyroid nodules and healthy controls. We included 32 studies from 6 articles. Overall, there were 463 PTC patients, 334 patients with benign thyroid nodules, and 104 healthy controls. The results showed that the summary sensitivity and specificity were 76% (95% confidence interval [CI]: 68%‒83%) and 86% (95% CI: 80%‒91%), respectively, and that the summary AUROC was 0.89 (95% CI: 0.86‒0.91), when serum miRNAs were used for discriminating between PTC patients and those with benign nodules. On the other hand, the summary sensitivity and specificity of serum miRNAs for discriminating between PTC patients and healthy controls were 82% (95% CI: 77%‒86%) and 84% (95% CI: 76%‒90%), respectively, and the summary AUROC was 0.89 (95% CI: 0.86‒0.92). We found that serum miRNAs have good diagnostic performance for the discrimination between patients with PTC and patients with benign nodules and healthy controls, and thus have considerable potential as novel minimally invasive tools for detecting PTC.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Yuping Chen, Bingtian Dong, Lichun Huang, Huibin Huang

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-05-17
Published 2022-10-23









