Evaluation of cerebrospinal fluid neurofilament light chain levels in multiple sclerosis and non-demyelinating diseases of the central nervous system: clinical and biochemical perspective
DOI:
https://doi.org/10.17305/bjbms.2021.7326Keywords:
Multiple sclerosis, neurofilament light chain, SIMOA, ELISAAbstract
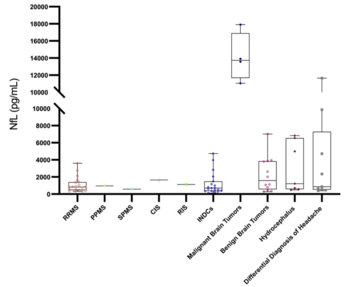
The neurofilament light chain (NfL) is a promising biomarker in the diagnosis, prognosis, and treatment response evaluation of neurological diseases. The aims of this study were to compare the cerebrospinal fluid (CSF) NfL levels in multiple sclerosis (MS) and certain non-demyelinating diseases of the central nervous system (NDCNS); to determine the relationship between clinical and radiological features and CSF NfL levels in patients with MS; and to compare the enzyme-linked immunosorbent assay (ELISA) and single molecule array (SIMOA) methods for NfL measurement using paired CSF and serum samples. We retrospectively analyzed the clinical data and performed NfL measurements in CSF and serum samples of newly diagnosed and treatment-naive patients with CNS diseases evaluated between 1 January 2019 and 1 January 2020. Eligible patients were divided into three groups: MS (n=23), differential diagnosis of MS (n=19), and NDCNS (n=42). First, we compared the CSF NfL levels among the three groups using the previously validated CSF ELISA assay. Next, we evaluated the relationship between CSF NfL levels and the clinical and radiological findings in MS group. Finally, we compared CSF and serum samples from patients of the MS groups (paired serum and CSF samples, n=19) using two different methods (ELISA and SIMOA). The CSF NfL level was the highest in the NDCNS group (1169.64 [535.92-5120.11] pg/mL, p=0.025). There was a strong positive correlation between the number of T2 lesions and CSF NfL level (r=0.786, p<0.001) in the MS group. There was excellent consistency between ELISA and SIMOA for CSF samples, but not for serum samples. Our results indicated that CSF NfL levels may also be used in the management of NDCNS and that SIMOA is the most reliable method for serum NfL determination.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2022 Burak Arslan, Gökçe Ayhan Arslan, Aslı Tuncer, Rana Karabudak, Aylin Sepici Dinçel

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-04-25
Published 2022-09-16









