The effect of sarcopenia on erlotinib therapy in patients with metastatic lung adenocarcinoma
DOI:
https://doi.org/10.17305/bjbms.2022.7147Keywords:
Lung cancer, sarcopenia, erlotinib, adenocarcinoma, prognosisAbstract
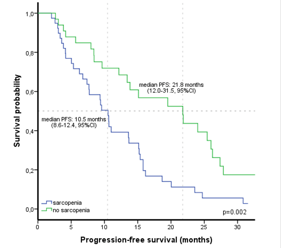
Erlotinib, a tyrosine kinase inhibitor, has been shown to improve the survival of patients with epidermal growth factor receptor (EGFR)-mutated non-small cell lung cancer. Sarcopenia is a status with increasing importance in lung cancer, and it may predict a poor prognosis. We aimed to evaluate the impact of sarcopenia on erlotinib therapy and prognosis in patients with EGFR-mutated (exon 19 or 21 L858R) metastatic lung adenocarcinoma. Sarcopenia was defined as skeletal muscle index ≤39 cm2/m2 for women and ≤55 cm2/m2 for men. The patient characteristics, inflammation parameters, clinical and survival outcomes of the erlotinib therapy were examined according to sarcopenia status. We also analyzed the erlotinib treatment-related toxicity. Seventy-two patients were included in our retrospective study, and the mean age of the patients was 63.7 years. A total of 39 (54.2%) patients were diagnosed with sarcopenia. Patients with sarcopenia had a poor prognosis and had a shorter median progression-free survival (PFS) than patients without sarcopenia (10.5 months vs. 21.8 months, p=0.002). Sarcopenia (HR 2.08) and C-reactive protein > 6.5 mg/L (HR 2.57) were determined as independent poor prognostic factors for PFS of erlotinib therapy. Treatment-related toxicity occurred in 34.7% of patients treated with erlotinib, and sarcopenia did not significantly affect treatment-related toxicity. We also found that sarcopenia significantly affected the response to erlotinib. The expected survival outcomes may be low when erlotinib therapy is used in patients with sarcopenia and metastatic lung adenocarcinoma. This study showed that survival and clinical outcomes could be better predicted by detecting sarcopenia in patients with lung cancer using erlotinib.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2022 Atakan Topcu, Akin Ozturk, Ismail Yurtsever, Mehmet Besiroglu, Ayse Irem Yasin, Haci Mehmet Turk, Mesut Seker

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-04-29
Published 2022-10-23









