The α7 nicotinic acetylcholine receptor agonist PNU-282987 ameliorates sepsis-induced acute kidney injury through CD4+CD25+ regulatory T cells in rats
DOI:
https://doi.org/10.17305/bjbms.2022.7111Keywords:
Selected:PNU-282987, sepsis, acute kidney injury, α7nAChR, Tregs, sepsis-induced acute kidney injury, SAKIAbstract
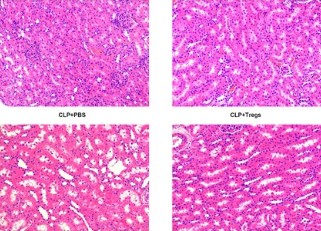
The ameliorative effects of α7 nicotinic acetylcholine receptor (α7nAChR) agonists have been demonstrated in acute kidney injury (AKI) caused by multiple stimulations. However, the ameliorative effect of α7nAChR on sepsis-induced acute kidney injury (SAKI) in the cecal ligation and puncture (CLP) model is unclear. Previous studies have demonstrated that α7nAChR is highly expressed on the surface of CD4+CD25+ regulatory T cells (Tregs). However, the role of Tregs in SAKI is unclear. We hypothesized that Tregs might play a role in the ameliorative effect of α7nAChR on SAKI. Hence, in this study, we determined the effects of PNU-282987 (a selective α7nAchR agonist) on SAKI and evaluated whether PNU-282987 would attenuate SAKI via regulating Tregs. Our study showed that immediate administration of PNU-282987 after CLP surgery in rats improved renal function, reduced levels of systemic inflammatory factors (tumor necrosis factor-α (TNF-α), interleukin-6 (IL-6), etc.), inflammatory cell infiltration and tubular apoptosis in renal tissues, and increased forkhead/winged helix transcription factor p3 (Foxp3) and cytotoxic T-lymphocyte-associated protein 4 (CTLA-4) expression indicating activated Tregs. Moreover, in in vitro experiments, isolated Tregs co-cultured with PNU-282987 also displayed enhanced expression of CTLA-4 and Foxp3. Furthermore, Tregs were co-cultured with PNU-282987 for 24 hours and then reinfused into rats through the tail vein immediately after CLP surgery, and a significant renal protective effect was observed 24 hours postoperatively. These results demonstrate that PNU-282987 exerts its renal protective effects on SAKI through activation of Tregs.
Citations
Downloads
Downloads
Additional Files
Published
License
Copyright (c) 2022 Xiaocui Shi, Juncong Li, Yuzhen Han, Jingyi Wang, Qingping Li, Yue Zheng, Wenxiong Li

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2022-05-08
Published 2022-10-23









