Nod-like receptor protein 3 inflammasome activation by Escherichia coli RNA induces transforming growth factor beta 1 secretion in hepatic stellate cells
DOI:
https://doi.org/10.17305/bjbms.2016.699Keywords:
Nod-like receptor protein 3, Inflammasome, Hepatic stellate cells, Transforming Growth factor β1Abstract
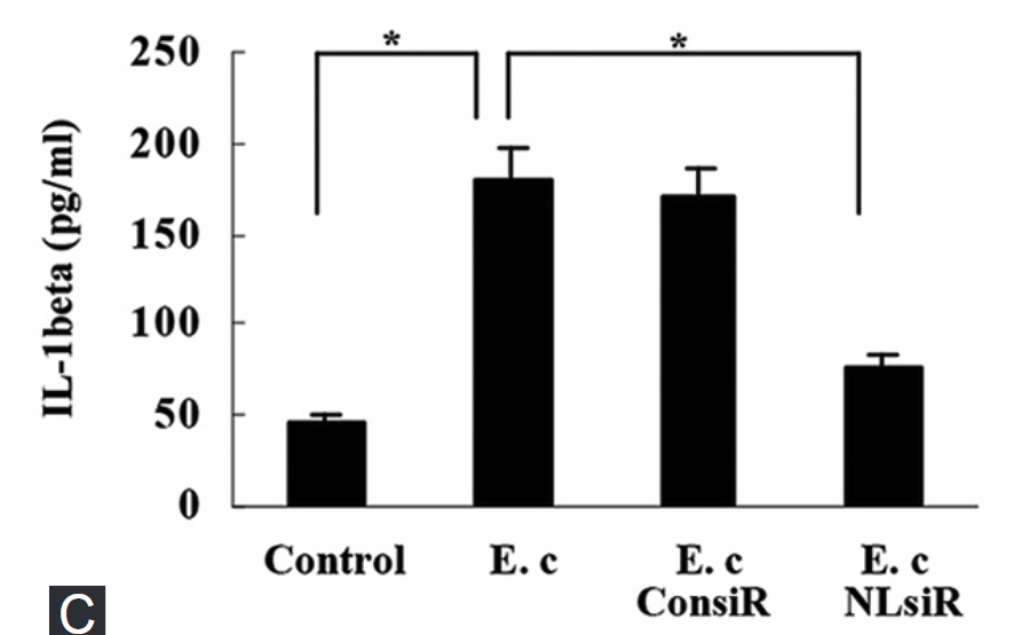
Nod-like receptor protein 3 (NLRP3) inflammasome has been implicated in alcoholic liver disease. Chronic alcohol consumption enhances gut permeability and causes microbial translocation. The present study explored the activation of the NLRP3 inflammasome by Escherichia coli RNA in hepatic stellate cells (HSCs), and the potential role of NLRP3 inflammasome in hepatic fibrosis. E. coli RNA transfection induced HSC-T6 cells to secrete and express mature interleukin-1 beta (IL-1β), which was abolished by NLRP3 siRNA pretreatment. In addition, E. coli RNA transfection enhanced caspase-1 expression, whereas reduced caspase-1 precursor (pro-caspase-1) expression. E. coli RNA-stimulated transforming growth factor beta 1 (TGF-β1) overproduction in HSC-T6 cells, which was blocked by recombinant IL-1 receptor antagonist (rIL-1Ra) or nuclear factor κB inhibitor BAY 11-7082. Furthermore, E. coli RNA-induced overexpression of pro-fibrogenic factors was suppressed by rIL-1Ra or TGF-β receptor inhibitor A83-01. These results demonstrate that E. coli RNA can stimulate NLRP3 inflammasome activation, which leads to excessive production of pro-fibrogenic factors, suggesting that NLRP3 inflammasome activation in HSCs may play a role in hepatic fibrosis.
Citations
Downloads
References
Franchi L, Eigenbrod T, Muñoz-Planillo R, Nuñez G. The inflammasome: A caspase-1-activation platform that regulates immune responses and disease pathogenesis. Nat Immunol 2009;10(3):241-7. http://dx.doi.org/10.1038/ni.1703.
Cassel SL, Joly S, Sutterwala FS. The NLRP3 inflammasome: A sensor of immune danger signals. Semin Immunol 2009;21(4):194-8. http://dx.doi.org/10.1016/j.smim.2009.05.002.
Strowig T, Henao-Mejia J, Elinav E, Flavell R. Inflammasomes in health and disease. Nature 2012;481(7381):278-86. http://dx.doi.org/10.1038/nature10759.
Franchi L, Muñoz-Planillo R, Núñez G. Sensing and reacting to microbes through the inflammasomes. Nat Immunol 2012;13(4):325-32. http://dx.doi.org/10.1038/ni.2231.
Szabo G, Bala S, Petrasek J, Gattu A. Gut-liver axis and sensing microbes. Dig Dis 2010;28(6):737-44. http://dx.doi.org/10.1159/000324281.
Schnabl B, Brenner DA. Interactions between the intestinal microbiome and liver diseases. Gastroenterology 2014;146(6):1513-24. http://dx.doi.org/10.1053/j.gastro.2014.01.020.
Rao RK, Seth A, Sheth P. Recent advances in alcoholic liver disease I. Role of intestinal permeability and endotoxemia in alcoholic liver disease. Am J Physiol Gastrointest Liver Physiol 2004;286(6):G881-4. http://dx.doi.org/10.1152/ajpgi.00006.2004.
Petrasek J, Bala S, Csak T, Lippai D, Kodys K, Menashy V, et al. IL-1 receptor antagonist ameliorates inflammasome-dependent alcoholic steatohepatitis in mice. J Clin Invest 2012;122(10):3476-89. http://dx.doi.org/10.1172/JCI60777.
Watanabe A, Sohail MA, Gomes DA, Hashmi A, Nagata J, Sutterwala FS, et al. Inflammasome-mediated regulation of hepatic stellate cells. Am J Physiol Gastrointest Liver Physiol 2009;296(6):G1248-57. http://dx.doi.org/10.1371/journal.ppat.1003330.
Son G, Hines IN, Lindquist J, Schrum LW, Rippe RA. Inhibition of phosphatidylinositol 3-kinase signaling in hepatic stellate cells blocks the progression of hepatic fibrosis. Hepatology 2009;50(5):1512-23. http://dx.doi.org/10.1002/hep.23186.
Franchi L, Kanneganti TD, Dubyak GR, Núñez G. Differential requirement of P2X7 receptor and intracellular K+ for caspase-1 activation induced by intracellular and extracellular bacteria. J Biol Chem 2007;282(26):18810-8. http://dx.doi.org/10.1074/jbc.M610762200.
Kanneganti TD, Lamkanfi M, Kim YG, Chen G, Park JH, Franchi L, et al. Pannexin-1-mediated recognition of bacterial molecules activates the cryopyrin inflammasome independent of Toll-like receptor signaling. Immunity 2007;26(4):433-43. http://dx.doi.org/10.1016/j.immuni.2007.03.008.
Kanneganti TD, Ozören N, Body-Malapel M, Amer A, Park JH, Franchi L, et al. Bacterial RNA and small antiviral compounds activate caspase-1 through cryopyrin/Nalp3. Nature 2006;440(7081):233-6. http://dx.doi.org/10.1038/nature04517.
Eigenbrod T, Franchi L, Muñoz-Planillo R, Kirschning CJ, Freudenberg MA, Núñez G, et al. Bacterial RNA mediates activation of caspase-1 and IL-1ß release independently of TLRs 3, 7, 9 and TRIF but is dependent on UNC93B. J Immunol 2012;189(1):328-36. http://dx.doi.org/10.4049/jimmunol.1103258.
Szabo G, Csak T. Inflammasomes in liver diseases. J Hepatol 2012;57(3):642-54. http://dx.doi.org/10.1016/j.jhep.2012.03.035.
Negash AA, Ramos HJ, Crochet N, Lau DT, Doehle B, Papic N, et al. IL-1ß production through the NLRP3 inflammasome by hepatic macrophages links hepatitis C virus infection with liver inflammation and disease. PLoS Pathog 2013;9(4):e1003330.
Wree A, Eguchi A, McGeough MD, Pena CA, Johnson CD, Canbay A, et al. NLRP3 inflammasome activation results in hepatocyte pyroptosis, liver inflammation, and fibrosis in mice. Hepatology 2014;59(3):898-910. http://dx.doi.org/10.1002/hep.26592.
Artlett CM. Inflammasomes in wound healing and fibrosis. J Pathol 2013;229(2):157-67. http://dx.doi.org/10.1002/path.4116.
Gasse P, Mary C, Guenon I, Noulin N, Charron S, Schnyder-Candrian S, et al. IL-1R1/MyD88 signaling and the inflammasome are essential in pulmonary inflammation and fibrosis in mice. J Clin Invest 2007;117(12):3786-99. http://dx.doi.org/10.1172/jci32285.
Dostert C, Pétrilli V, Van Bruggen R, Steele C, Mossman BT, Tschopp J. Innate immune activation through Nalp3 inflammasome sensing of asbestos and silica. Science 2008;320(5876):674-7. http://dx.doi.org/10.1126/science.1156995.
Cassel SL, Eisenbarth SC, Iyer SS, Sadler JJ, Colegio OR, Tephly LA, et al. The Nalp3 inflammasome is essential for the development of silicosis. Proc Natl Acad Sci U S A 2008;105(26):9035-40. http://dx.doi.org/10.1073/pnas.0803933105.
Artlett CM, Sassi-Gaha S, Rieger JL, Boesteanu AC, Feghali-Bostwick CA, Katsikis PD. The inflammasome activating caspase 1 mediates fibrosis and myofibroblast differentiation in systemic sclerosis. Arthritis Rheum 2011;63(11):3563-74. http://dx.doi.org/10.1002/art.30568.
Shinde AV, Frangogiannis NG. Fibroblasts in myocardial infarction: A role in inflammation and repair. J Mol Cell Cardiol 2014;70:74-82. http://dx.doi.org/10.1016/j.yjmcc.2013.11.015.
Wree A, McGeough MD, Peña CA, Schlattjan M, Li H, Inzaugarat ME, et al. NLRP3 inflammasome activation is required for fibrosis development in NAFLD. J Mol Med (Berl) 2014;92(10):1069-82. http://dx.doi.org/10.1007/s00109-014-1170-1.
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2016 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2015-10-15
Published 2016-01-14









