Three-dimensional telomere profiles in papillary thyroid cancer variants: A pilot study
DOI:
https://doi.org/10.17305/bjbms.2021.6639Keywords:
Papillary thyroid cancer, noninvasive follicular thyroid neoplasm with papillary-like nuclear features, follicular adenoma, 3D telomere profiles, NIFTP, FTA, PTCAbstract
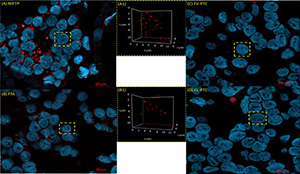
Besides the two main histologic types of papillary thyroid carcinoma (PTC), the classical PTC (CL-PTC) and the follicular variant PTC (FV-PTC), several other variants are described. The encapsulated FV-PTC variant was recently reclassified as noninvasive follicular thyroid neoplasm with papillary-like nuclear features (NIFTP) due to its similarities to benign lesions. Specific molecular signatures, however, are still unavailable. It is well known that improper DNA repair of dysfunctional telomeres may cause telomere-related genome instability. The mechanisms involved in the damaged telomere repair processing may lead to detrimental outcomes, altering the three-dimensional (3D) nuclear telomere and genome organization in cancer cells. This pilot study aimed to evaluate whether a specific 3D nuclear telomere architecture might characterize NIFTP, potentially distinguishing it from other PTC histologic variants. Our findings demonstrate that 3D telomere profiles of CL-PTC and FV-PTC were different from NIFTP and that NIFTP more closely resembles follicular thyroid adenoma (FTA). NIFTP has longer telomeres than CL-PTC and FV-PTC samples, and the telomere length of NIFTP overlaps with that of the FTA histotype. In contrast, there was no association between BRAF expression and telomere length in all tested samples. These preliminary findings reinforce the view that NIFTP is closer to non-malignant thyroid nodules and confirm that PTC features short telomeres.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2021 Aline Rangel-Pozzo, Tinuccia Dettori, Daniela Virginia Frau, Federica Etzi, John Gartner, Garbor Fisher, Roberta Vanni, Sabine Mai, Paola Caria

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2021-11-11
Published 2022-06-01









