Development and validation of a novel pre-operative comprehensive prognostic score in esophageal squamous cell carcinoma
DOI:
https://doi.org/10.17305/bjbms.2021.6350Keywords:
Prognostic score, esophageal squamous cell carcinoma, squamous cell carcinoma antigen, c-reactive protein to albumin ratio, neutrophil to lymphocyte ratio, fibrinogen, cancer-specific survivalAbstract
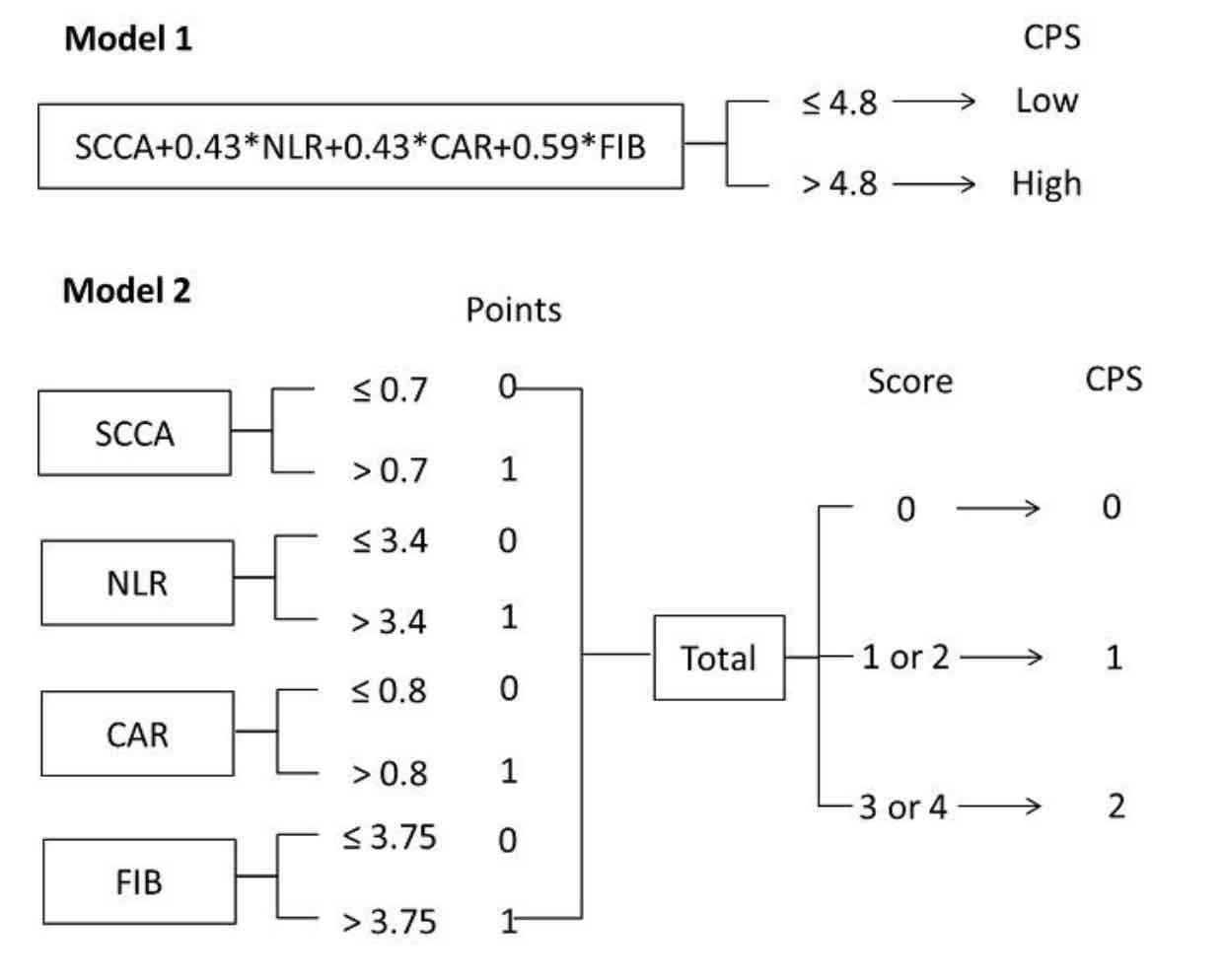
We herein propose a novel integrative score based on inflammatory and nutritional score, coagulation indicator and tumor marker, named comprehensive prognostic score (CPS), to predict postoperative survival in resectable esophageal squamous cell carcinoma (ESCC). We also aimed to establish and validate a nomogram based on CPS and other clinical features for individual survival prediction. A total of 490 resectable ESCC patients were randomly divided into either a training or validation cohort at a ratio of 7:3 for retrospective analysis. The CPS, based on squamous cell carcinoma antigen (SCCA), C-reactive protein to albumin ratio (CAR), neutrophil to lymphocyte ratio (NLR), and fibrinogen (FIB), was divided into two models to verify its prognostic value. The predictive model of CPS-based nomogram was established and validated in two cohorts. Patients with CPS low group in model 1 had better 5-year cancer-specific survival (CSS) than those in CPS high group (50.7% vs. 17.8%, P<0.001). For model 2, the 5-year CSS for CPS 0, 1 and 2 were 75.0%, 38.9% and 13.3%, respectively (P<0.001). CPS was confirmed as an independent prognostic score in both models. The CPS-based nomogram can accurately and effectively predict survival in resected ESCC. The CPS is a novel, simple, and effective predictor in resectable ESCC. Moreover, CPS has a potential independent prognostic value in predicting postoperative survival, which can accurately and effectively predict individual survival in resectable ESCC.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2021 Jifeng Feng, Liang Wang, Xun Yang, Qixun Chen

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2021-10-12
Published 2022-06-01









