Correlation between advanced glycation end-products and the expression of fatty inflammatory factors in type II diabetic cardiomyopathy
DOI:
https://doi.org/10.17305/bjbms.2015.619Keywords:
Cardiomyopathy, adiponectin, leptin, advanced glycation end-productsAbstract
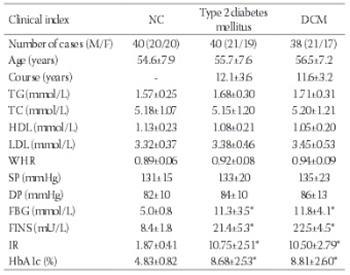
Diabetic cardiomyopathy (DCM) is one of the most severe complications of diabetes without a clear pathogenesis. Th is study investigated the adiponectin (APN) and leptin levels in type II DCM, as well as their correlation with advanced glycation end-products (AGEs). From 2011–2013, 78 type II diabetes mellitus (T2DM) cases (40–65 years old) in the Taian region were randomly selected. Based on the results of colour Doppler ultrasonography and coronary angiography, the cases were divided into a simple T2DM group (40 cases) and a DCM group (38 cases). Forty healthy subjects were used as normal control (NC). An enzyme-linked immunosorbent assay was performed to determine the levels of fa tty infl ammatory factors such as APN, leptin and AGEs, and a correlation analysis was conducted. In the T2DM group, the APN levels were decreased but the leptin and AGE levels were signifi cantly increased compared to the NC group. In the DCM group, the APN levels were decreased but the leptin and AGE levels were signifi cantly increased (P<0.01) compared to the T2DM group. Th e AGE levels were positively correlated with disease progression and with fasting plasma glucose levels, glycated haemoglobin, insulin resistance and leptin, but were negatively correlated with APN levels. Additionally, the APN and leptin levels were independently related to the AGE levels. Fatty infl ammatory factors play a signifi cant role in the progression of both simple T2DM and DCM. Th e results of this study revealed the pathogenesis of DCM and indicated the potential signifi cance of AGEs in DCM prevention and treatment.
Citations
Downloads
References
Hamby RI, Zonerich S, Sherman L. Diabetic cardiomyopathy. JAMA 1974;229:1749-1754. http://dx.doi.org/10.1001/jama.1974.03230510023016.
Yang W, Lu J, Weng J, Jia W, Ji L, Xiao J, et al. Prevalence of diabetes among men and women in china. N Engl J Med 2010;362(12):1090-1101. http://dx.doi.org/10.1056/NEJMoa0908292.
Brownlee M. Th e pathobiology of diabetic complications: a unifying mechanism. Diabetes 2005;54(6):1615-1625. http://dx.doi.org/10.2337/diabetes.54.6.1615.
Aronson D. Cross-linking of glycated collagen in the pathogenesis of arterial and myocardial stiff ening of ageing
and diabetes. J Hypertens 2003;21(1):3-12. http://dx.doi.org/10.1097/00004872-200301000-00002.
Yudkin JS. Insulin resistance and the metabolic syndrome-or the pitfalls of epidemiology. Diabetologia 2007;50(8):1576-1586. http://dx.doi.org/10.1007/s00125-007-0711-3.
Kajitani N, shikata K, Nakamura A, Nakatou T, Hiramatsu M, Makino H. Microinfl ammation is a common risk factor for progression of nephropathy and atherosclerosis in Japanese patients with type 2 diabetes. Diabetes Res Clin Pract 2010;88(2):171-176. http://dx.doi.org/10.1016/j.diabres.2010.01.012.
Grunenfelder J, Nminiati D, Murata S, Falk V, Hoyt EG, Robbins RC. Up-regulation of Bcl-2 through hyperbaric pressure transfection of TGF-β1: ameliorates is chemi-areperfusion injury in rat cardiac alloGrafts.
J Heart Lung Transplant 2004;21(2):244-250. http://dx.doi.org/10.1016/S1053-2498(01)00377-1.
Sun Y, ZhangJ, Zhang JQ, Ramires FJ. Local angiotensin II and transforming growth factor-beta1 in renal fi brosis of rats. Hypertension 2004;35(5):1078-1084. http://dx.doi.org/10.1161/01.HYP.35.5.1078.
Li Q, Li B, Wang X, Leri A, Jana KP, Liu Y, et al. Overexpression of insulin-like growth factor-1 in mice protects from myocyte death after infarction, attenuating ventricular dilation, wall stress, and cardiac
hypertrophy. J Clin Invest 1997;100(8):991-1999. http://dx.doi.org/10.1172/jci119730.
Unger RH. Minireview: weapons of lean body mass destruction: the role of ectopic lipids in the metabolic syndrome. Endocrinology 2003;144(12):5159-5165. http://dx.doi.org/10.1210/en.2003-0870.
Li J, Su S, Zong X. Analysis of the association between adiponectin, adiponectin receptor 1 and diabetic cardiomyopathy. Exp Th er Med 2014;7(4):1023-1027. http://dx.doi.org/10.3892/etm.2014.1539.
Huang H, Song CY, Du DY, Liu Y, Lai XH, Jiang F, et al. Effect of intensive insulin treatment on oxidative stress level at early stage of diabetic cardio myopathy in elderly patients. Chinese Journal of Multiple Organ Diseases in the Elderly 2012;11(6):442-444.
Vlassara H. Protein glycation in the kidney: role in diabetes and ageing. Kidney Int 1996;49(6):1795-1804. http://dx.doi.org/10.1038/ki.1996.270.
Freeman DJ, Norrie J, Caslake MJ. C-reactive protein is an independent predictor of risk for the development of diabetes in the West of Scotland Coronary Prevention Study. Diabetes 2002;(5):1596-1600 http://dx.doi.org/10.2337/diabetes.51.5.1596.
Gonon AT, Widegren U, Bulhak A, Salehzadeh F, Persson J, Sjöquist PO, et al. Adiponectin protects againstmyocardial ischaemia-reperfusion injury via AMP-activated proteinkinase, Akt,
and nitric oxide. Cardiovasc Res 2008;78(1):116-122 http://dx.doi.org/10.1093/cvr/cvn017.
Tsai WC, Lin CC, Chen JY, Huang YY, Lee CH, Li WT, et al. Association of adiponectin with procollagentype I carboxyterminal propeptide in non-diabetic essential hypertension. Blood Press 2008;17(4):233-238. http://dx.doi.
org/10.1080/08037050802308895.
Lieb W, Sullivan LM, Aragam J, Harris TB, Roubenoff R, Benjamin EJ, et al. Relation of serum leptin with cardiac mass and left atrial dimension in individuals >70 years of age. Am J Cardiol 2009;104(4):602-605. http://dx.doi.org/10.1016/j.amjcard.2009.04.026.
Karmazyn M, Gan XT, Rajapurohitam V. Th e potential contribution of circulating and locally produced leptin to cardiac hypertrophy and failure. Can J Physiol Pharmacol 2013;91(11):883-888. http://dx.doi.org/10.1139/cjpp-2013-0057.
Hegab Z, Gibbons S, Neyses L, Mamas MA. Role of advanced glycation end products in cardiovascular disease. World cardiol 2012;4(4): 90-102. http://dx.doi.org/10.4330/wjc.v4.i4.90.
Giacco F, Brownlee M. Oxidative stress and diabetic complications. Circ Res 2010;107:1058-1070. http://dx.doi.org/10.1161/CIRCRESAHA.110.223545.
Snijdex MB, Heine RJ, Seidell JC, Bouter LM, Stehouwer CD, Nijpels G, et al. Associations of adiponectin levels with incident impaired glucose metabolism and type 2 diabetes in o1dely Men and
Women: the Hoom study. Diabetes Care 2006;29(11):2498-2503. http://dx.doi.org/10.2337/dc06-0952.
Wang CY, Zhang W, Zhou YW. Adiponectin reduced myocardial fribrosis in diabetic cardiomyopathy through anti-oxidative stress. International Journal of Laboratory Medicine 2010;31:780-782.
Karmazyn M, Gan XT, Rajapurohitam V. Th e potential contribution of circulating and locally produced leptin to cardiac hypertrophy and failure. Can J Physiol Pharmacol 2013;91(11):883-888. http://dx.doi.org/10.1139/cjpp-2013-0057.
Unger RH, Scherer PE. Gluttony, sloth and the metabolic syndrome:a roadmap to lipotoxicity. Trends Endocrinol Metab 2010;21(6):345-352. http://dx.doi.org/10.1016/j.tem.2010.01.009.
Hilzendeger AM, Morais RL, Todiras M, Plehm R, da Costa Goncalves A, Qadri F, et al. Leptin regulates ACE activity in mice. J Mol Med (Berl) 2010;88(9):899-907. http://dx.doi.org/10.1007/s00109-010-0649-7.
Rahmouni K. Obesity, sympathetic overdrive, and hypertension: the leptin connection. Hypertension 2010;55(4):844-845. http://dx.doi.org/10.1161/HYPERTENSIONAHA.109.148932.
Chiu HC, Kovacs A, Blanton RM, Han X, Courtois M, Weinheimer CJ, et al. Transgenic expression of fatty acid transport protein 1 in the heart causes lipotoxic cardiomyopathy. Circ Res 2005;96(2):225-233. http://dx.doi.org/10.1161/01.RES.0000154079.20681.B9.
Finck BN. Th e role of the peroxisome proliferator activated receptor alpha pathway in pathological remodinseling of the diabetic heart. Current Opinion in Clinic Nutrition & Metabolic Care 2004;7(4):391-396. http://dx.doi.org/10.1097/01.mco.0000134371.70815.32.
Downloads
Additional Files
Published
How to Cite
Accepted 2015-08-18
Published 2015-10-25









