Endothelial loss during the surgical procedure in saphenous veins harvested by open and endoscopic techniques in coronary artery bypass surgery
DOI:
https://doi.org/10.17305/bjbms.2020.4656Keywords:
Endothelial cells, saphenous vein, endoscopic harvesting, open harvesting, coronary artery bypass graftingAbstract
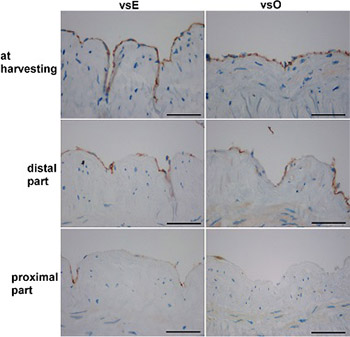
The patency of the vein graft in coronary artery bypass grafting could be dependent on the conventional open (vsO) or endoscopic (vsE) harvesting and on the hypoxic damage of endothelial cells. We aimed to evaluate both surgical techniques according to endothelial loss that occurs in the time between harvesting and implantation. Twenty-six saphenous veins were divided into vsO (n = 16) and vsE (n = 10) group. Three samples were taken from each vein. The first sample was taken after removal, the second before implantation of the distal part, and the third before the implantation of the proximal part, and they were stained with HE, Movat, and immunohistochemically with CD31. A significant loss of endothelial cells within both groups was found at the time of implantation of the distal and the proximal part of the vein graft compared to the endothelial cells at the time of harvesting. There were no significant differences in the endothelial loss between vsE and vsO groups at the time of harvesting and at the time before the implantation of the distal part. A higher number of endothelial cells was found in vsE group compared to vsO group at the time just before the implantation of the proximal part. The comparison of the implanted portions of vsE and vsO grafts to mammary arteries revealed a significant loss of endothelial cells only in vsO graft. We conclude that, at the time of implantation, the endothelial layer of the vein graft harvested endoscopically is more preserved than of the vein graft harvested openly.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2020-03-18
Published 2020-11-02









