High MTHFR promoter methylation levels in men confer protection against ischemic stroke
DOI:
https://doi.org/10.17305/bjbms.2020.4636Keywords:
Ischemic stroke, MTHFR, methylation, Hcy, hypertensionAbstract
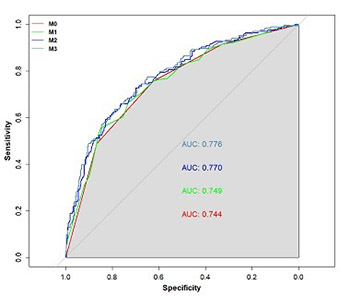
The MTHFR gene encodes methylenetetrahydrofolate reductase required for the metabolism of homocysteine (Hcy) – a previously reported independent risk factor for ischemic stroke (IS). In this study, we first aimed to clarify the association between DNA methylation levels in the MTHFR promoter and the risk of IS, followed by the analysis of potential interactions between environmental factors and DNA methylation levels that affect IS risk. We recruited 164 patients with hypertension and IS (case group) and 345 age-matched and sex-matched patients with hypertension only (control group). Demographic and clinical information was obtained using questionnaires, and blood samples were collected for biochemical analyses. Fluorescence quantitative methylation-specific PCR (qMSP) was used to detect MTHFR promoter methylation levels. A logistic regression analysis was performed to determine the relationship between environmental factors, MTHFR promoter methylation levels, and IS risk. We finally generated a receiver operating characteristic curve to determine whether MTHFR promoter methylation levels can predict IS. The mean MTHFR methylation levels in the case group (8.10 ± 6.14) were significantly lower than those in the control group (17.44 ± 3.16; p < 0.05). MTHFR promoter methylation levels were also lower in patients with plasma Hcy levels ≥15 μmol/L (10.65 ± 4.05) than in those with Hcy levels <15 μmol/L (16.74 ± 4.26, p < 0.001). Finally, we found that MTHFR hypermethylation is a protective factor for IS, particular in men (OR in men: 0.07; 95% CI: 0.02–0.16; p < 0.001). Further, sex and MTHFR promoter methylation levels exhibited a preliminary interaction effect on IS risk. These results indicate that MTHFR promoter methylation status might have diagnostic value in IS.
Citations
Downloads
Downloads
Additional Files
Published
How to Cite
Accepted 2020-04-29
Published 2020-11-02









