Comparison of the effects of preoperative melatonin or vitamin C administration on postoperative analgesia
DOI:
https://doi.org/10.17305/bjbms.2019.4379Keywords:
Melatonin, morphine consumption, postoperative analgesia, vitamin CAbstract
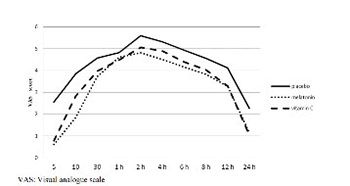
The analgesic benefit of melatonin and vitamin C as primary or adjuvant agents has been reported in various studies; however, their analgesic effects in the treatment of postoperative pain remain unclear. Thus, we aimed to evaluate the effect of single preoperative dose of oral melatonin or vitamin C administration on postoperative analgesia. In this study, we recruited 165 adult patients undergoing elective major abdominal surgery under general anesthesia. Patients were randomly divided into three equal (n = 55) groups. One hour before surgery, patients received orally melatonin (6 mg) in group M, vitamin C (2 g) in group C, or a placebo tablet in group P. Pain, sedation, patient satisfaction, total morphine consumption from a patient-controlled analgesia device, supplemental analgesic requirement, and the incidence of nausea and vomiting were recorded throughout 24 h after surgery. The mean pain score and total morphine consumption were found significantly lower in both M and C groups compared with group P (p < 0.001). There were no significant differences between group M and C with respect to pain scores (p = 0.117) and total morphine consumption (p = 0.090). Patients requested less supplemental analgesic and experienced less nausea and vomiting in groups M and C compared with group P. In conclusion, preoperative oral administration of 6 mg melatonin or 2 g vitamin C led to a reduction in pain scores, total morphine consumption, supplemental analgesic requirement, and the incidence of nausea and vomiting compared with placebo.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2019-08-06
Published 2020-02-05









