Induction of epithelial-mesenchymal transition (EMT) and Gli1 expression in head and neck squamous cell carcinoma (HNSCC) spheroid cultures
DOI:
https://doi.org/10.17305/bjbms.2018.3243Keywords:
Head and neck cell carcinoma, cancer stem cells, CSCs, epithelial-mesenchymal transition, EMT, tumor microenvironment, hypoxia, Gli1Abstract
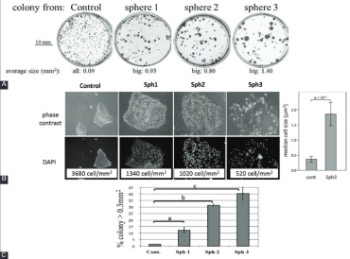
Tumor microenvironment provides a specialized niche in which a population of stem-like cells is enriched and contributes to cancer progression. Moreover, cancer stem cell (CSC) phenotype has been associated with epithelial-mesenchymal transition (EMT). Here we investigated the effect of tumor microenvironment on the phenotypic characteristics of head and neck cancer cells and expression of CSC markers using a three-dimensional (3D), spheroid, culture system of CAL33 cell line from human tongue squamous cell carcinoma. CAL33 cells derived from 2D monolayer cultures were grown in spheroid cultures containing serum-free medium (epidermal growth factor [EGF], fibroblast growth factor [FGF], and insulin). Adherent CAL33 cells from spheroids or standard control cultures were grown in the presence/absence of serum in combination with hypoxia/normoxia. Markers of EMT, CSC, and hypoxia were analyzed either by Western blotting, immunofluorescence, or reverse transcription quantitative PCR. Spheroid cultures showed hypoxic microenvironment (high carbonic anhydrase IX [CAIX] expression), mesenchymal-like characteristics (reduced E-cadherin and increased vimentin and N-cadherin expression, presence of larger colonies comprised of larger, spread cells with lower density), and increased expression of the CSC marker glioma-associated oncogene homolog 1 (Gli1). These effects were recapitulated in serum-free adherent CAL33 cells maintained for prolonged periods in hypoxia (1% O2) but, in contrast, were completely abolished by the presence of serum. Overall, we found that a combination of hypoxia, EGF and FGF was essential to induce the EMT in adherent CAL33 cell cultures. The addition of serum rapidly reverts the EMT of cells, affects CSC phenotype and, thus, prevents the detection of such cells in tumor cell lines.
Citations
Downloads
References
Batlle E, Clevers H. Cancer stem cells revisited. Nat Med 2017;23(10):1124-34. https://doi.org/10.1038/nm.4409.
Visvader JE, Lindeman GJ. Cancer stem cells in solid tumours: Accumulating evidence and unresolved questions. Nat Rev Cancer 2008;8(10):755-68. https://doi.org/10.1038/nrc2499.
Singh SK, Clarke ID, Terasaki M, Bonn VE, Hawkins C, Squire J, et al. Identification of a cancer stem cell in human brain tumors. Cancer Res 2003;63(18):5821-8.
Dionne LK, Driver ER, Wang XJ. Head and neck cancer stem cells: From identification to tumor immune network. J Dent Res 2015;94(11):1524-31. https://doi.org/10.1177/0022034515599766.
Cui Y, Cui CA, Yang ZT, Ni WD, Jin Y, Xuan YH. Gli1 expression in cancer stem-like cells predicts poor prognosis in patients with lung squamous cell carcinoma. Exp Mol Pathol 2017;102(2):347-53. https://doi.org/10.1016/j.yexmp.2017.03.004.
Chung CH, Dignam JJ, Hammond ME, Klimowicz AC, Petrillo SK, Magliocco A, et al. Glioma-associated oncogene family zinc finger 1 expression and metastasis in patients with head and neck squamous cell carcinoma treated with radiation therapy (RTOG 9003). J Clin Oncol 2011;29(10):1326-34. https://doi.org/10.1200/JCO.2010.32.3295.
Bielecka ZF, Maliszewska-Olejniczak K, Safir IJ, Szczylik C, Czarnecka AM. Three-dimensional cell culture model utilization in cancer stem cell research. Biol Rev Camb Philos Soc 2017;92(3):1505-20. https://doi.org/10.1111/brv.12293.
Kimlin LC, Casagrande G, Virador VM. In vitro three-dimensional (3D) models in cancer research: An update. Mol Carcinog 2013;52(3):167-82. https://doi.org/10.1002/mc.21844.
Fujii H, Honoki K, Tsujiuchi T, Kido A, Yoshitani K, Takakura Y. Sphere-forming stem-like cell populations with drug resistance in human sarcoma cell lines. Int J Oncol 2009;34(5):1381-6. https://doi.org/10.3892/ijo_00000265.
Ponti D, Costa A, Zaffaroni N, Pratesi G, Petrangolini G, Coradini D, et al. Isolation and in vitro propagation of tumorigenic breast cancer cells with stem/progenitor cell properties. Cancer Res 2005;65(13):5506-11. https://doi.org/10.1158/0008-5472.CAN-05-0626.11.
Diepenbruck M, Christofori G. Epithelial-mesenchymal transition (EMT) and metastasis: Yes, no, maybe? Curr Opin Cell Biol 2016;43:7-13. https://doi.org/10.1016/j.ceb.2016.06.002.
Thiery JP, Acloque H, Huang RYJ, Nieto MA. Epithelial-mesenchymal transitions in development and disease. Cell 2009;139(5):871-90. https://doi.org/10.1016/j.cell.2009.11.007.
Bierie B, Pierce SE, Kroeger C, Stover DG, Pattabiraman DR, Thiru P, et al. Integrin-β4 identifies cancer stem cell-enriched populations of partially mesenchymal carcinoma cells. Proc Natl Acad Sci USA 2017;114(12):E2337-46. https://doi.org/10.1073/pnas.1618298114.
Gioanni J, Fischel JL, Lambert JC, Demard F, Mazeau C, Zanghellini E, et al. Two new human tumor cell lines derived from squamous cell carcinomas of the tongue: Establishment, characterization and response to cytotoxic treatment. Eur J Cancer Clin Oncol 1988;24(9):1445-55. https://doi.org/10.1016/0277-5379(88)90335-5.
Nagafuchi A, Takeichi M, Tsukita S. The 102 kd cadherin-associated protein: Similarity to vinculin and posttranscriptional regulation of expression. Cell 1991;65(5):849-57. https://doi.org/10.1016/0092-8674(91)90392-C.
Sedlakova O, Svastova E, Takacova M, Kopacek J, Pastorek J, Pastorekova S. Carbonic anhydrase IX, a hypoxia-induced catalytic component of the pH regulating machinery in tumors. Front Physiol 2014;4:400. https://doi.org/10.3389/fphys.2013.00400.
Mani SA, Guo W, Liao MJ, Eaton EN, Ayyanan A, Zhou AY, et al. The epithelial-mesenchymal transition generates cells with properties of stem cells. Cell 2008;133(4):704-15. https://doi.org/10.1016/j.cell.2008.03.027.
Ino Y, Gotoh M, Sakamoto M, Tsukagoshi K, Hirohashi S. Dysadherin, a cancer-associated cell membrane glycoprotein, down-regulates E-cadherin and promotes metastasis. Proc Natl Acad Sci USA 2002;99(1):365-70. https://doi.org/10.1073/pnas.012425299.
Beavon IR. Regulation of E-cadherin: Does hypoxia initiate the metastatic cascade? Mol Pathol 1999;52(4):179-88. https://doi.org/10.1136/mp.52.4.179.
Shiraishi A, Tachi K, Essid N, Tsuboi I, Nagano M, Kato T, et al. Hypoxia promotes the phenotypic change of aldehyde dehydrogenase activity of breast cancer stem cells. Cancer Sci 2017;108(3):362-72. https://doi.org/10.1111/cas.13147.
Hong X, Chedid K, Kalkanis SN. Glioblastoma cell line-derived spheres in serum‑containing medium versus serum-free medium: A comparison of cancer stem cell properties. Int J Oncol 2012;41(5):1693-700. https://doi.org/10.3892/ijo.2012.1592.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2018-04-24
Published 2018-11-07









