Immune cells and vasa vasorum in the tunica media of atherosclerotic coronary arteries
DOI:
https://doi.org/10.17305/bjbms.2018.2951Keywords:
Atherosclerosis, coronary arteries, tunica media, T cells, B cells, macrophages, immune privilege, vasa vasorumAbstract
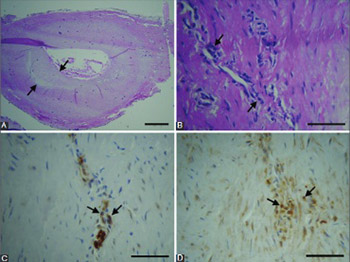
In coronary artery disease (CAD), the disruption of the tunica media immune privilege manifests as increased leukocyte infiltration and the formation of vasa vasorum. We aimed to characterize the immune privilege status of the tunica media in human coronary arteries (CAs) with atherosclerotic plaques, by comparing the abundance and composition of immune-cell infiltrates within the individual arterial-wall layers, and by evaluating vasa vasorum neovascularization of the tunica media. The tissue samples were obtained from 36 symptomatic patients with diffuse CAD (aged 60–72 years) who underwent coronary endarterectomy. T and B cells, macrophages and endothelial cells in the CAs were detected by immunohistochemistry. Morphological analysis of CAs showed significant atherosclerotic changes in all specimens. In the media, we observed damage and loss of smooth muscle cells, destruction of the extracellular matrix architecture, and fibrosis. There were 43.3% of immune cells in the intima, 50% in the adventitia, and 6.7% in the media. In the media, 51.1% of the immune cells were T cells (p ˂ 0.001 compared to B cells and macrophages; ANOVA, Scheffe post hoc analysis), 23.5% were B cells, and 25.4% were macrophages. The number of vasa vasorum in the media was 1 in 38.9% of CAs, 2–3 in 36.1%, and ≥4 in 25% of CAs. Our results indicate that, in atherosclerotic CAs, the immune privilege of the media is disrupted by the infiltration of T and B cells, macrophages, and the presence of vasa vasorum.
Citations
Downloads
References
Goodarzynejad H, Boroumand M, Behmanesh M, Ziaee S, Jalali A. Cholesteryl ester transfer protein gene polymorphism (I405V) and premature coronary artery disease in an Iranian population. Bosn J Basic Med Sci 2016;16(2):114-20. https://doi.org/10.17305/bjbms.2016.942.
Tosheska K, Labudovic D, Jovanova S, Jaglikovski B, Alabakovska S. Cholesteryl ester transfer protein, low density lipoprotein particle size and intima media thickness in patients with coronary heart disease. Bosn J Basic Med Sci 2011;1(3):169-73. https://doi.org/10.17305/bjbms.2011.2569.
Satilmis S, Celik O, Biyik I, Ozturk D, Celik K, Akın F, et al. Association between serum vitamin D levels and subclinical coronary atherosclerosis and plaque burden/composition in young adult population. Bosn J Basic Med Sci 2015;15(1):67-72. https://doi.org/10.17305/bjbms.2015.238.
Tellides G, Pober JS. Inflammatory and immune responses in the arterial media. Circ Res 2015;116(2):312-22. https://doi.org/10.1161/CIRCRESAHA.116.301312.
Wilens SL, McCluskey RT. The comparative filtration properties of excised arteries and veins. Am J Med Sci 1952;224(5):540-7.
https://doi.org/10.1097/00000441-195211000-00009.
Dal Canto AJ, Swanson PE, O'Guin AK, Speck SH, Virgin HW. IFN-gamma action in the media of the great elastic arteries, a novel immunoprivileged site. J Clin Invest 2001;107(2):R15-22. https://doi.org/10.1172/JCI11540.
Cuffy MC, Silverio AM, Qin L, Wang Y, Eid R, Brandacher G, et al. Induction of indoleamine 2,3-dioxygenase in vascular smooth muscle cells by interferon-gamma contributes to medial immunoprivilege. J Immunol 2007;179(8):5246-54. https://doi.org/10.4049/jimmunol.179.8.5246.
Munn DH, Mellor AL. Indoleamine 2,3 dioxygenase and metabolic control of immune responses. Trends Immunol 2013;34(3):137-43. https://doi.org/10.1016/j.it.2012.10.001.
Lebastchi AH, Khan SF, Qin L, Li W, Zhou J, Hibino N, et al. Transforming growth factor beta expression by human vascular cells inhibits interferon gamma production and arterial media injury by alloreactive memory T cells. Am J Transplant 2011;11(11):2332-41. https://doi.org/10.1111/j.1600-6143.2011.03676.x.
Tellides G, Tereb DA, Kirkiles, Smith NC, Kim RW, Wilson JH, et al. Interferon-gamma elicits arteriosclerosis in the absence of leukocytes. Nature 2000;403(6766):207-11. https://doi.org/10.1038/35003221.
Zhang P, Manes TD, Pober JS, Tellides G. Human vascular smooth muscle cells lack essential costimulatory molecules to activate allogeneic memory T cells. Arterioscler Thromb Vasc Biol 2010;30(9):1795-1801. https://doi.org/10.1161/ATVBAHA.109.200758.
Al-Soudi A, Kaaij MH, Tas SW. Endothelial cells: From innocent bystanders to active participants in immune responses. Autoimmun Rev 2017;16(9):951-62. https://doi.org/10.1016/j.autrev.2017.07.008.
Geiringer E. The mural coronary. Am Heart J 1951;41(3):359-68. https://doi.org/10.1016/0002-8703(51)90036-1.
Zhang Y, Cliff WJ, Schoefl GI, Higgins G. Immunohistochemical study of intimal microvessels in coronary atherosclerosis. Am J Pathol 1993;143(1):164-72.
Ho-Tin-Noe B, Le Dall J, Gomez D, Louedec L, Vranckx R, El-Bouchtaoui M, et al. Early atheroma-derived agonists of peroxisome proliferator-activated receptor-gamma trigger intramedial angiogenesis in a smooth muscle cell dependent manner. Circ Res 2011;109(9):1003-14. https://doi.org/10.1161/CIRCRESAHA.110.235390.
Nicoletti A, Khallou-Laschet J, Guedj K, Clement M, Gaston AT, Morvan M, et al. L19. Lymphoid neogenesis in vascular chronic inflammation. Presse Med 2013;42(4 Pt 2):558-60. https://doi.org/10.1016/j.lpm.2013.01.018.
Chistiakov DA, Orekhov AN, Bobryshev YV. Immune-inflammatory responses in atherosclerosis: Role of an adaptive immunity mainly driven by T and B cells. Immunobiology 2016;221(9):1014-2033. https://doi.org/10.1016/j.imbio.2016.05.010.
Virmani R, Kolodgie FD, Burke AP, Farb A, Schwartz SM. Lessons from sudden coronary death: A comprehensive morphological classification scheme for atherosclerotic lesions. Arterioscler Thromb Vasc Biol 2000;20(5):1262-75. https://doi.org/10.1161/01.ATV.20.5.1262.
Medscape [Internet]. Ladich ER, Virmani R, Kolodgie F, Otsuka F: Atherosclerosis Pathology; 2016 [2017 September 12]. Available from: https://reference.medscape.com/article/1612610-overview.
Hagemeijer MC, van Oosterhout MF, van Wichen DF, van Kuik J, Siera-de Koning E, Gmelig Meyling FH, et al. T cells in cardiac allograft vasculopathy are skewed to memory Th-1 cells in the presence of a distinct Th-2 population. Am J Transplant 2008;8(5):1040-50. https://doi.org/10.1111/j.1600-6143.2008.02198.x.
Billingham ME. Cardiac transplant atherosclerosis. Transplant Proc 1987;19(4 Suppl 5):19-25.
van der Wal AC, Das PK, Bentz van de Berg D, van der Loos CM, Becker AE. Atherosclerotic lesions in humans. In situ immunophenotypic analysis suggesting an immune mediated response. Lab Invest 1989;61(2):166-70.
Emeson EE, Robertson AL Jr. T lymphocytes in aortic and coronary intimas. Their potential role in atherogenesis. Am J Pathol 1988;130(2):369-76.
Yang X, Coriolan D, Murthy V, Schultz K, Golenbock DT, Beasley D. Proinflammatory phenotype of vascular smooth muscle cells: Role of efficient Toll-like receptor 4 signaling. Am J Physiol Heart Circ Physiol 2005;289(3):H1069-76. https://doi.org/10.1152/ajpheart.00143.2005.
Yang X, Murthy V, Schultz K, Tatro JB, Fitzgerald KA, Beasley D. Toll-like receptor 3 signaling evokes a proinflammatory and proliferative phenotype in human vascular smooth muscle cells. Am J Physiol Heart Circ Physiol 2006;291(5):H2334-43. https://doi.org/10.1152/ajpheart.00252.2006.
Ahmad U, Ali R, Lebastchi AH, Qin L, Lo SF, Yakimov AO, et al. IFN-gamma primes intact human coronary arteries and cultured coronary smooth muscle cells to double-stranded RNA- and self-RNA-induced inflammatory responses by upregulating TLR3 and melanoma differentiation-associated gene 5. J Immunol 2010;185(2):1283-94. https://doi.org/10.4049/jimmunol.0902283.
Cole JE, Navin TJ, Cross AJ, Goddard ME, Alexopoulou L, Mitra AT, et al. Unexpected protective role for Toll-like receptor 3 in the arterial wall. Proc Natl Acad Sci U S A 2011;108(6):2372-7. https://doi.org/10.1073/pnas.1018515108.
Yang X, Coriolan D, Schultz K, Golenbock DT, Beasley D. Toll-like receptor 2 mediates persistent chemokine release by Chlamydia pneumoniae-infected vascular smooth muscle cells. Arterioscler Thromb Vasc Biol 2005;25(11):2308-14. https://doi.org/10.1161/01.ATV.0000187468.00675.a3.
Sun J, Ding Y. NOD2 agonist promotes the production of inflammatory cytokines in VSMC in synergy with TLR2 and TLR4 agonists. ScientificWorldJournal 2012;2012:607157. https://doi.org/10.1100/2012/607157.
Pober JS, Tellides G. Participation of blood vessel cells in human adaptive immune responses. Trends Immunol 2012;33(1):49-57. https://doi.org/10.1016/j.it.2011.09.006.
Canducci F, Saita D, Foglieni C, Piscopiello MR, Chiesa R, Colombo A, et al. Cross-reacting antibacterial auto-antibodies are produced within coronary atherosclerotic plaques of acutecoronary syndrome patients. PLoS One 2012;7(8):e42283. https://doi.org/10.1371/journal.pone.0042283.
Ketelhuth DF, Hansson GK. Adaptive response of T and B cells in atherosclerosis. Circ Res 2016;118(4):668-78. https://doi.org/10.1161/CIRCRESAHA.115.306427.
Hansson GK, Libby P. The immune response in atherosclerosis: A double-edged sword. Nat Rev Immunol 2006;6(7):508-19. https://doi.org/10.1038/nri1882.
Mohanta SK, Yin C, Peng L, Srikakulapu P, Bontha V, Hu D, et al. Artery tertiary lymphoid organs contribute to innate and adaptive immune responses in advanced mouse atherosclerosis. Circ Res 2014;114(11):1772-87. https://doi.org/10.1161/CIRCRESAHA.114.301137.
Gössl M, Rosol M, Malyar NM, Fitzpatrick LA, Beighley PE, Zamir M, et al. Functional anatomy and hemodynamic characteristics of vasa vasorum in the walls of porcine coronary arteries. Anat Rec A Discov Mol Cell Evol Biol 2003;272(2):526-37. https://doi.org/10.1002/ar.a.10060.
Moreno PR, Purushothaman KR, Fuster V, Echeverri D, Truszczynska H, Sharma SK, et al. Plaque neovascularization is increased in ruptured atherosclerotic lesions of human aorta: Implications for plaque vulnerability. Circulation 2004;110(14):2032-8 https://doi.org/10.1161/01.CIR.0000143233.87854.23.
Tang PC, Yakimov AO, Teesdale MA, Coady MA, Dardik A, Elefteriades JA, et al. Transmural inflammation by interferon-gamma-producing T cells correlates with outward vascular remodeling and intimal expansion of ascending thoracic aortic aneurysms. FASEB J 2005;19(11):1528-30. https://doi.org/10.1096/fj.05-3671fje.
Moulton KS, Vakili K, Zurakowski D, Soliman M, Butterfield C, Sylvin E, et al. Inhibition of plaque neovascularization reduces macrophage accumulation and progression of advanced atherosclerosis. Proc Natl Acad Sci U S A 2003;100(8):4736-41. https://doi.org/10.1073/pnas.0730843100.
Eliska O, Eliskova M, Miller AJ. The absence of lymphatics in normal and atherosclerotic coronary arteries in man: A morphologic study. Lymphology 2006;39(2):76-83.
Downloads
Additional Files
Published
How to Cite
Accepted 2018-02-19
Published 2018-08-01









