Berberine alleviates oxidative stress in rats with osteoporosis through receptor activator of NF-kB/receptor activator of NF-kB ligand/osteoprotegerin (RANK/RANKL/OPG) pathway
DOI:
https://doi.org/10.17305/bjbms.2017.2596Keywords:
Osteoporosis, oxidative stress, Wistar rats, berberine, OPG, osteoprotegerin, RANK, RANKL, receptor activator of nuclear factor kappa-B ligandAbstract
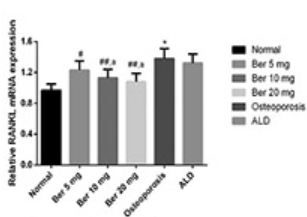
Previous studies suggested that oxidative stress is related to the onset and development of osteoporosis. Moreover, it was demonstrated that berberine has a protective effect against oxidative stress-induced injuries. In this study, we aimed to investigate the effect and mechanism of action of berberine on rats with induced osteoporosis. Sixty 8-week-old female Wistar rats were randomly divided into the following 6 groups: control saline-treated, osteoporosis saline-treated, 3 osteoporosis berberine-treated groups (Ber 5, 10, and 20 mg/kg/body weight, respectively), and osteoporosis alendronate-treated (ALD) group. Osteoporosis was induced by bilateral ovariectomy. All treatments were performed for 8 weeks. The bone mineral density (BMD), serum alkaline phosphatase (ALP), osteocalcin, calcium, phosphorus, superoxide dismutase (SOD), methylenedioxyamphetamine (MDA), and glutathione peroxidase (GSH-Px) level was determined in the rat femur tissue. The gene and protein expression of osteoprotegerin (OPG) and receptor activator of nuclear factor kappa-B ligand (RANKL) was analyzed by quantitative reverse transcription PCR and Western blot, respectively. The BMD, SOD and GSH⁃Px levels, and the expression of OPG were significantly lower in osteoporosis compared to control group (all p < 0.05). The serum levels of osteocalcin, ALP, and MDA, and the expression of RANKL were significantly higher in osteoporosis compared to control group (all p < 0.05). Berberine, especially the high doses of berberine, effectively increased SOD, GSH⁃Px, and OPG levels as well as decreased serum osteocalcin, ALP, MDA and RANKL levels in berberine-treated osteoporosis groups (all p < 0.05). To conclude, oxidative stress may promote the development of osteoporosis in rats through the RANK/RANKL/OPG pathway. The antioxidative effect of berberine reduces the development of osteoporosis in rats to some extent.
Citations
Downloads
References
Zhen D, Liu L, Guan C, Zhao N, Tang X. High prevalence of vitamin D deficiency among middle-aged and elderly individuals in northwestern China: Its relationship to osteoporosis and lifestyle factors. Bone 2015;71:1-6. https://doi.org/10.1016/j.bone.2014.09.024.
Chen SJ, Liao WC, Huang KH, Lin CL, Tsai WC, Kung PT, et al. Chronic obstructive pulmonary disease and allied conditions is a strong independent risk factor for osteoporosis and pathologic fractures: A population-based cohort study. QJM 2015;108(8):633-40. https://doi.org/10.1093/qjmed/hcv012.
Weaver CM, Alexander DD, Boushey CJ, Dawson-Hughes B, Lappe JM, LeBoff MS, et al. Calcium plus vitamin D supplementation and risk of fractures: An updated meta-analysis from the National Osteoporosis Foundation. Osteoporos Int 2016;27(1):367-76.
https://doi.org/10.1007/s00198-015-3386-5.
Mody N, Parhami F, Sarafian TA, Demer LL. Oxidative stress modulates osteoblastic differentiation of vascular and bone cells. Free Radic Biol Med 2001;31(4):509-19.
https://doi.org/10.1016/S0891-5849(01)00610-4.
Gyoneva L, Hovell CB, Pewowaruk RJ, Dorfman KD, Segal Y, Barocas VH. Cell-matrix interaction during strain-dependent remodelling of simulated collagen networks. Interface Focus 2016;6(1):20150069. https://doi.org/10.1098/rsfs.2015.0069.
Kim BJ, Shin KO, Kim H, Ahn SH, Lee SH, Seo CH, et al. The effect of sphingosine-1-phosphate on bone metabolism in humans depends on its plasma/bone marrow gradient. J Endocrinol Invest 2016;39(3):297-303. https://doi.org/10.1007/s40618-015-0364-x.
Vääräniemi J, Halleen JM, Kaarlonen K, Ylipahkala H, Alatalo SL, Andersson G, et al. Intracellular machinery for matrix degradation in bone-resorbing osteoclasts. J Bone Miner Res 2004;19(9):1432-40. https://doi.org/10.1359/JBMR.040603.
Lee NK, Choi YG, Baik JY, Han SY, Jeong DW, Bae YS, et al. A crucial role for reactive oxygen species in RANKL-induced osteoclast differentiation. Blood 2005;106(3):852-9. https://doi.org/10.1182/blood-2004-09-3662.
Pellegrini GG, Morales CC, Wallace TC, Plotkin LI, Bellido T. Avenanthramides prevent osteoblast and osteocyte apoptosis and induce osteoclast apoptosis in vitro in an Nrf2-independent manner. Nutrients 2016;8(7). pii: E423. https://doi.org/10.3390/nu8070423.
Pietschmann P, Mechtcheriakova D, Meshcheryakova A, Föger-Samwald U, Ellinger I. Immunology of osteoporosis: A mini-review. Gerontology 2016;62(2):128-37. https://doi.org/10.1159/000431091.
Lee IA, Hyun YJ, Kim DH. Berberine ameliorates TNBS-induced colitis by inhibiting lipid peroxidation, enterobacterial growth and NF-κB activation. Eur J Pharmacol 2010;648(1-3):162-70. https://doi.org/10.1016/j.ejphar.2010.08.046.
Li P, Ren J, Duan C, Lin C, Liu J. Effects of four components of Rhizoma Corydalis on anoxia and peroxidation injuries in neonatal cardiomyocytes. [Article in Chinese]. Zhongguo Zhong Yao Za Zhi 2010;35(1):84-8.
Huang Z, Chen S, Zhang G, Xu S, Huang W, Han Y, et al. Protective effects of berberine and phentolamine on myocardial reoxygenation damage. Chin Med Sci J 1992;7(4):221-5.
Zhou L, Song F, Liu Q, Yang M, Zhao J, Tan R, et al. Berberine sulfate attenuates osteoclast differentiation through RANKL induced NF-κB and NFAT pathways. Int J Mol Sci 2015;16(11):27087-96. https://doi.org/10.3390/ijms161125998.
Zheng B, Li G, Chen WC, Deasy BM, Pollett JB, Sun B, et al. Human myogenic endothelial cells exhibit chondrogenic and osteogenic potentials at the clonal level. J Orthop Res 2013;31(7):1089-95. https://doi.org/10.1002/jor.22335.
Fu C, Xu D, Wang CY, Jin Y, Liu Q, Meng Q, et al. Alpha-lipoic acid promotes osteoblastic formation in H2O2-treated MC3T3-E1 cells and prevents bone loss in ovariectomized rats. J Cell Physiol 2015;230(9):2184-2201. https://doi.org/10.1002/jcp.24947.
Dougall WC, Glaccum M, Charrier K, Rohrbach K, Brasel K, De Smedt T, et al. RANK is essential for osteoclast and lymph node development. Genes Dev 1999;13(18):2412-24. https://doi.org/10.1101/gad.13.18.2412.
Joo JH, Huh JE, Lee JH, Park DR, Lee Y, Lee SG, et al. A novel pyrazole derivative protects from ovariectomy-induced osteoporosis through the inhibition of NADPH oxidase. Sci Rep 2016;6:22389. https://doi.org/10.1038/srep22389.
Hemshekhar M, Thushara RM, NaveenKumar SK, Manoj P, Sundaram MS, Kemparaju K, et al. Bone degeneration, inflammation and secondary complications of arthritis: Potential targets and their natural inhibitors. Mini Rev Med Chem 2017. https://doi.org/10.2174/1389557517666170315144233. [Epub ahead of print].
Abdollahi M, Larijani B, Rahimi R, Salari P. Role of oxidative stress in osteoporosis. Therapy 2005;2(5):787-96. DOI: 10.1586/14750708.2.5.787.
Zhao H, Xu H, Qiao S, Lu C, Wang G, Liu M, et al. Boldine isolated from Litsea cubeba inhibits bone resorption by suppressing the osteoclast differentiation in collagen-induced arthritis. Int Immunopharmacol 2017;51:114-23. DOI: 10.1016/j.intimp.2017.08.013.
Kobayashi Y, Udagawa N, Takahashi N. Action of RANKL and OPG for osteoclastogenesis. Crit Rev Eukaryot Gene Expr 2009;19(1):61-72. https://doi.org/10.1615/CritRevEukarGeneExpr.v19.i1.30.
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2017 Bosnian Journal of Basic Medical Sciences

This work is licensed under a Creative Commons Attribution 4.0 International License.
How to Cite
Accepted 2017-10-22
Published 2017-11-20









