5-fluorouracil, leucovorin, and irinotecan (FOLFIRI) as a third-line chemotherapy treatment in metastatic gastric cancer, after failure of fluoropyrimidine, platinum, anthracycline, and taxane
DOI:
https://doi.org/10.17305/bjbms.2017.2258Keywords:
Chemotherapy, metastatic gastric cancer, modified FOLFIRI, third-line therapy, prognosis, 5-fluorouracil, leucovorin, irinotecanAbstract
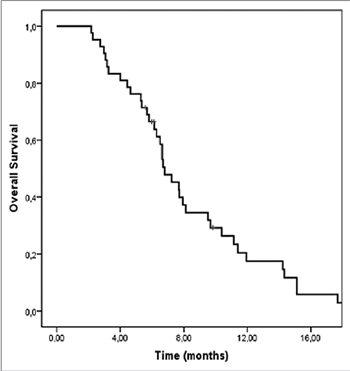
Studies on the effects of third-line chemotherapy (CT) in advanced gastric cancer (GC) patients are still scarce. The aim of this study was to evaluate the efficacy and safety of the modified 5-fluorouracil, leucovorin, and irinotecan (mFOLFIRI) regimen as a third-line CT in metastatic GC patients, after failure of fluoropyrimidine, platinum, anthracycline, and taxane. After failure of first- and second-line therapies, 42 patients received third-line FOLFIRI (180 mg/m² irinotecan and 400 mg/m² leucovorin administered concomitantly as a 90-minute intravenous (IV) infusion on day 1, followed by a 400 mg/m² 5-fluorouracil IV bolus then 2600 mg/m² continuous infusion over 46 hours), between January 2009 and December 2015. FOLFIRI was administered for a median of 6 cycles (range 4-12 cycles). Eight patients achieved partial response, while 13 patients showed stable disease, resulting in the overall response rate (ORR) of 19% and disease control rate (DCR) of 50%. The most frequent grade 3-4 hematological and non-hematological toxicities were neutropenia (14.2%) and diarrhea (7.1%). The median progression-free survival (PFS) and overall survival (OS) from the start of third-line CT were 3.8 months (95% confidence interval [CI], 3.0-4.5) and 6.8 months (95% CI, 5.6-7.9), respectively. According to the multivariate analysis, two factors were independently predictive of the poor OS: >2 regions of metastasis (relative risk [RR], 2.6; 95% CI, 1.3-5.4) and a high level of carcinoembryonic antigen [CEA] (RR, 3.4; 95% CI, 1.6-7.4). In conclusion, FOLFIRI was well tolerated as third-line CT and showed promising PFS and OS in advanced GC patients, after failure of fluoropyrimidine, platinum, anthracycline, and taxane.
Citations
Downloads
References
Ferlay J, Soerjomataram I, Dikshit R, Eser S, Mathers C, Rebelo M, et al. Cancer incidence and mortality worldwide: Sources, methods and major patterns in GLOBOCAN 2012. Int J Cancer 2015;136(5):E359-86. https://doi.org/10.1002/ijc.29210.
Wagner AD, Grothe W, Haerting J, Kleber G, Grothey A, Fleig WE. Chemotherapy in advanced gastric cancer: A systematic review and meta-analysis based on aggregate data. J Clin Oncol 2006;24(18):2903-9. https://doi.org/10.1200/JCO.2005.05.0245.
Wagner AD, Unverzagt S, Grothe W, Kleber G, Grothey A, Haerting J, et al. Chemotherapy for advanced gastric cancer. Cochrane Database Syst Rev 2010;3:CD004064. https://doi.org/10.1002/14651858.CD004064.pub3.
Van Cutsem E, Moiseyenko VM, Tjulandin S, Majlis A, Constenla M, Boni C, et al. Phase III study of docetaxel and cisplatin plus fluorouracil compared with cisplatin and fluorouracil as first-line therapy for advanced gastric cancer: A report of the V325 study group. J Clin Oncol 2006;24(31):4991-7. https://doi.org/10.1200/JCO.2006.06.8429.
Chi Y, Ren JH, Yang L, Cui CX, Li JL, Wang JW. Phase II clinical study on the modified DCF regimen for treatment of advanced gastric carcinoma. Chin Med J (Engl) 2011;124(19):2997-3002.
Keskin S, Yildiz I, Sen F, Aydogan F, Kilic L, Ekenel M, et al. Modified DCF (mDCF) regimen seems to be as effective as original DCF in advanced gastric cancer (AGC). Clin Transl Oncol 2013;15(5):403-8. https://doi.org/10.1007/s12094-012-0942-8.
Kang JH, Lee SI, Lim DH, Park KW, Oh SY, Kwon HC, et al. Salvage chemotherapy for pretreated gastric cancer: A randomized phase III trial comparing chemotherapy plus best supportive care with best supportive care alone. J Clin Oncol 2012;30(13):1513-8. https://doi.org/10.1200/JCO.2011.39.4585.
Kim HS, Kim HJ, Kim SY, Kim TY, Lee KW, Baek SK, et al. Second-line chemotherapy versus supportive cancer treatment in advanced gastric cancer: A meta-analysis. Ann Oncol 2013;24(11):2850-4. https://doi.org/10.1093/annonc/mdt351.
Thuss-Patience PC, Kretzschmar A, Bichev D, Deist T, Hinke A, Breithaupt K, et al. Survival advantage for irinotecan versus best supportive care as second-line chemotherapy in gastric cancer - A randomised phase III study of the Arbeitsgemeinschaft Internistische Onkologie (AIO). Eur J Cancer 2011;47(15):2306-14. https://doi.org/10.1016/j.ejca.2011.06.002.
Hironaka S, Ueda S, Yasui H, Nishina T, Tsuda M, Tsumura T, et al. Randomized, open-label, phase III study comparing irinotecan with paclitaxel in patients with advanced gastric cancer without severe peritoneal metastasis after failure of prior combination chemotherapy using fluoropyrimidine plus platinum: WJOG 4007 trial. J Clin Oncol 2013;31(35):4438-44. https://doi.org/10.1200/JCO.2012.48.5805.
Wilke H, Muro K, Van Cutsem E, Oh SC, Bodoky G, Shimada Y, et al. Ramucirumab plus paclitaxel versus placebo plus paclitaxel in patients with previously treated advanced gastric or gastro-oesophageal junction adenocarcinoma (RAINBOW): A double-blind, randomised phase 3 trial. Lancet Oncol 2014;15(11):1224-35. https://doi.org/10.1016/S1470-2045(14)70420-6.
Hess LM, Michael D, Mytelka DS, Beyrer J, Liepa AM, Nicol S. Chemotherapy treatment patterns, costs, and outcomes of patients with gastric cancer in the United States: A retrospective analysis of electronic medical record (EMR) and administrative claims data. Gastric Cancer 2016;19(2):607-15. https://doi.org/10.1007/s10120-015-0486-z.
Kang EJ, Im SA, Oh DY, Han SW, Kim JS, Choi IS, et al. Irinotecan combined with 5-fluorouracil and leucovorin third-line chemotherapy after failure of fluoropyrimidine, platinum, and taxane in gastric cancer: Treatment outcomes and a prognostic model to predict survival. Gastric Cancer 2013;16(4):581-9. https://doi.org/10.1007/s10120-012-0227-5.
Lee JH, Kim SH, Oh SY, Lee S, Lee H, Lee HJ, et al. Third-line docetaxel chemotherapy for recurrent and metastatic gastric cancer. Korean J Intern Med 2013;28(3):314-21. http://192.168.2.1:8090/httpclient.html.
Assersohn L, Brown G, Cunningham D, Ward C, Oates J, Waters JS, et al. Phase II study of irinotecan and 5-fluorouracil/leucovorin in patients with primary refractory or relapsed advanced oesophageal and gastric carcinoma. Ann Oncol 2004;15(1):64-9. https://doi.org/10.1093/annonc/mdh007.
Kim SG, Oh SY, Kwon HC, Lee S, Kim JH, Kim SH, et al. A phase II study of irinotecan with bi-weekly, low-dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as salvage therapy for patients with advanced or metastatic gastric cancer. Jpn J Clin Oncol 2007;37(10):744-9. https://doi.org/10.1093/jjco/hym103.
Seo MD, Lee KW, Lim JH, Yi HG, Kim DY, Oh DY, et al. Irinotecan combined with 5-fluorouracil and leucovorin as second-line chemotherapy for metastatic or relapsed gastric cancer. Jpn J Clin Oncol 2008;38(9):589-95. https://doi.org/10.1093/jjco/hyn078.
Sym SJ, Ryu MH, Lee JL, Chang HM, Kim TW, Lee SS, et al. Salvage chemotherapy with biweekly irinotecan, plus 5-fluorouracil and leucovorin in patients with advanced gastric cancer previously treated with fluoropyrimidine, platinum, and taxane. Am J Clin Oncol 2008;31(2):151-6. https://doi.org/10.1097/COC.0b013e31815878a2.
Eisenhauer EA, Therasse P, Bogaerts J, Schwartz LH, Sargent D, Ford R, et al. New response evaluation criteria in solid tumours: Revised RECIST guideline (version 1.1). Eur J Cancer 2009;45(2):228-47. https://doi.org/10.1016/j.ejca.2008.10.026.
Ji SH, Lim DH, Yi SY, Kim HS, Jun HJ, Kim KH, et al. A retrospective analysis of second-line chemotherapy in patients with advanced gastric cancer. BMC Cancer 2009;9:110. https://doi.org/10.1186/1471-2407-9-110.
Shim HJ, Yun JY, Hwang JE, Bae WK, Cho SH, Chung IJ. Prognostic factor analysis of third-line chemotherapy in patients with advanced gastric cancer. Gastric Cancer 2011;14(3):249-56. https://doi.org/10.1007/s10120-011-0032-6.
Shah MA. Update on metastatic gastric and esophageal cancers. J Clin Oncol 2015;33(16):1760-9. https://doi.org/10.1200/JCO.2014.60.1799.
Kim BG, Oh SY, Kwon HC, Lee S, Lee DM, Kim SG, et al. A phase II study of irinotecan with biweekly, low dose leucovorin and bolus and continuous infusion 5-fluorouracil (modified FOLFIRI) as first line therapy for patients with recurrent or metastatic gastric cancer. Am J Clin Oncol 2010;33(3):246-50. DOI: 10.1097/COC.0b013e3181a650d4.
Kwon HJ, Park MI, Park SJ, Moon W, Kim SE, Lee HW, et al. Efficacy and safety of FOLFIRI after failure of FOLFOX-4 in advanced gastric cancer. Korean J Gastroenterol 2015;66(1):10-6. https://doi.org/10.4166/kjg.2015.66.1.10.
Pasquini G, Vasile E, Caparello C, Vivaldi C, Musettini G, Lencioni M, et al. Third-line chemotherapy with irinotecan plus 5-fluorouracil in Caucasian metastatic gastric cancer patients. Oncology 2016;91(6):311-6. https://doi.org/10.1159/000443962.
Godai TI, Oshima T, Numata M, Fukahori M, Sato T, Makino H, et al. Clinical efficacy and safety of CPT-11+CDDP therapy as third-line chemotherapy for advanced and recurrent gastric cancer. [Article in Japanese] Gan To Kagaku Ryoho 2011;38(6):945-9.
Lee MJ, Hwang IG, Jang JS, Choi JH, Park BB, Chang MH, et al. Outcomes of third-line docetaxel-based chemotherapy in advanced gastric cancer who failed previous oxaliplatin-based and irinotecan-based chemotherapies. Cancer Res Treat 2012;44(4):235-41. https://doi.org/10.4143/crt.2012.44.4.235.
Moon YW, Rha SY, Jeung HC, Kim C, Hong MH, Chang H, et al. Outcomes of multiple salvage chemotherapy for advanced gastric cancer: Implications for clinical practice and trial design. Cancer Chemother Pharmacol 2010;66(4):797-805.
https://doi.org/10.1007/s00280-010-1295-z.
Shimoyama R, Yasui H, Boku N, Onozawa Y, Hironaka S, Fukutomi A, et al. Weekly paclitaxel for heavily treated advanced or recurrent gastric cancer refractory to fluorouracil, irinotecan, and cisplatin. Gastric Cancer 2009;12(4):206-11.
https://doi.org/10.1007/s10120-009-0524-9.
Nishimura T, Iwasa S, Nagashima K, Okita N, Takashima A, Honma Y, et al. Irinotecan monotherapy as third-line treatment for advanced gastric cancer refractory to fluoropyrimidines, platinum, and taxanes. Gastric Cancer 2017;20(4):655-62.
https://doi.org/10.1007/s10120-016-0670-9.
Catalano V, Graziano F, Santini D, D’Emidio S, Baldelli AM, Rossi D, et al. Second-line chemotherapy for patients with advanced gastric cancer: Who may benefit? Br J Cancer 2008;99(9):1402-7. https://doi.org/10.1038/sj.bjc.6604732.
Louvet C, Carrat F, Mal F, Mabro M, Beerblock K, Vaillant JC, et al. Prognostic factor analysis in advanced gastric cancer patients treated with hydroxyurea, leucovorin, 5-fluorouracil, and cisplatin (HLFP regimen). Cancer Invest 2003;21(1):14-20.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-08-16
Published 2018-05-20









