Analysis of pulmonary surfactant by Fourier transform infrared spectroscopy after exposure to sevoflurane and isoflurane
DOI:
https://doi.org/10.17305/bjbms.2016.1680Keywords:
Pulmonary surfactant, isoflurane, sevoflurane, Fourier transform infrared, spectroscopy, principal component analysisAbstract
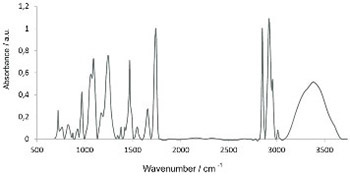
Pulmonary surfactant, consisting primarily of phospholipids and four surfactant-specific proteins, is among the first structures that is exposed to inhalation anesthetics. Consequently, changes of pulmonary surfactant due to this exposure could cause respiratory complications after long anesthetic procedures. Fourier transform infrared (FTIR) spectroscopy was used to explore the effects of two inhalation anesthetics, sevoflurane and isoflurane, on a commercially available pulmonary surfactant. The research was primarily focused on the effect of anesthetics on the lipid component of the surfactant. Four different concentrations of anesthetics were added, and the doses were higher from the low clinical doses typically used. Recorded spectra were analyzed using principal component analysis, and the Student’s t-test was performed to confirm the results. The exposure to both anesthetics induced similar changes, consistent with the increase of the anesthetic concentration. The most pronounced effect was on the hydrophilic head group of phospholipids, which is in agreement with the disruption of the hydrogen bond, caused by the anesthetics. A change in the band intensities of CH2 stretching vibrations, indicative of a disordering effect of anesthetics on the hydrophobic tails of phospholipids, was also observed. Changes induced by isoflurane appear to be more pronounced than those induced by sevoflurane. Furthermore, our results suggest that FTIR spectroscopy is a promising tool in studying anesthetic effects on pulmonary surfactant.
Citations
Downloads
References
Weibel ER. Morphometry of the human lung. Berlin, Göttingen, Heidelberg: Springer Verlag; 1963.
Bourbon JR. Pulmonary surfactant: biochemical, functional, regulatory and clinical concepts. Boca Raton, FL: CRC Press; 1991.
Bellisola G, Sorio C. Infrared spectroscopy and microscopy in cancer research and diagnosis. Am J Cancer Res 2012;2(1):1-21.
Kaneshina S, Kamaya H, Ueda I. Transfer of anesthetics and alcohols into ionic surfactant micelles in relation to depression of krafft-point and critical micelle concentration and interfacial interaction of anesthetics. J Colloid Interface Sci 1981;83(2):589-98. http://dx.doi.org/10.1016/0021-9797(81)90353-2.
Yoshida T, Takahashi K, Ueda I. Molecular orientation of volatile anesthetics at the binding surface: 1H - And 19F-NMR studies of submolecular affinity. Biochim Biophys Acta 1989;985(3):331-3. http://dx.doi.org/10.1016/0005-2736(89)90421-5.
Tsai YS, Ma SM, Kamaya H, Ueda I. Fourier transform infrared studies on phospholipid hydration: Phosphate-oriented hydrogen bonding and its attenuation by volatile anesthetics. Mol Pharmacol 1987;31(6):623-30.
Tsai YS, Ma SM, Nishimura S, Ueda I. Infrared spectra of phospholipid membranes: Interfacial dehydration by volatile anesthetics and phase transition. Biochim Biophys Acta 1990;1022(2):245-50. http://dx.doi.org/10.1016/0005-2736(90)90120-D.
Baber J, Ellena JF, Cafiso DS. Distribution of general anesthetics in phospholipid bilayers determined using 2H NMR and 1H-1H NOE spectroscopy. Biochemistry 1995;34(19):6533-9. http://dx.doi.org/10.1021/bi00019a035.
Tu K, Tarek M, Klein ML, Scharf D. Effects of anesthetics on the structure of a phospholipid bilayer: Molecular dynamics investigation of halothane in the hydrated liquid crystal phase of dipalmitoylphosphatidylcholine. Biophys J 1998;75(5):2123-34. http://dx.doi.org/10.1016/S0006-3495(98)77655-6.
Koubi L, Tarek M, Klein ML, Scharf D. Distribution of halothane in a dipalmitoylphosphatidylcholine bilayer from molecular dynamics calculations. Biophys J 2000;78(2):800-11. http://dx.doi.org/10.1016/S0006-3495(00)76637-9.
North C, Cafiso DS. Contrasting membrane localization and behavior of halogenated cyclobutanes that follow or violate the Meyer-Overton hypothesis of general anesthetic potency. Biophys J 1997;72(4):1754-61.
http://dx.doi.org/10.1016/S0006-3495(97)78821-0.
Yoshino A, Murate K, Yoshida T, Okabayashi H, Krishna PR, Kamaya H, et al. Surface-oriented saturable binding of halothane with micelles: Paramagnetic relaxation of 19F-NMR spin-lattice relaxation rate and gas chromatography studies. J Colloid Interface Sci 1994;166(2):375-82. http://dx.doi.org/10.1006/jcis.1994.1308.
Yoshino A, Yoshida T, Okabayashi H, Kamaya H, Ueda I. 19F and 1H NMR and NOE study on halothane-micelle interaction: Residence location of anesthetic molecules. J Colloid Interface Sci 1998;198(2):319-22. http://dx.doi.org/10.1006/jcis.1997.5322.
Craig NC, Bryant GJ, Levin IW. Effects of halothane on dipalmitoylphosphatidylcholine liposomes: A Raman spectroscopic study. Biochemistry 1987;26(9):2449-58. http://dx.doi.org/10.1021/bi00383a008.
Rüdiger M, Tölle A, Meier W, Rüstow B. Naturally derived commercial surfactants differ in composition of surfactant lipids and in surface viscosity. Am J Physiol Lung Cell Mol Physiol 2005;288(2):L379-83. http://dx.doi.org/10.1152/ajplung.00176.2004.
Rak S, De Zan T, Stefulj J, Kosovic M, Gamulin O, Osmak M. FTIR spectroscopy reveals lipid droplets in drug resistant laryngeal carcinoma cells through detection of increased ester vibrational bands intensity. Analyst 2014;139(13):3407-15. http://dx.doi.org/10.1039/c4an00412d.
Gasper R, Dewelle J, Kiss R, Mijatovic T, Goormaghtigh E. IR spectroscopy as a new tool for evidencing antitumor drug signatures. Biochim Biophys Acta 2009;1788(6):1263-70. http://dx.doi.org/10.1016/j.bbamem.2009.02.016.
Gaigneaux A, Ruysschaert JM, Goormaghtigh E. Cell discrimination by attenuated total reflection - Fourier transform infrared spectroscopy: The impact of preprocessing of spectra. Appl Spectrosc 2006;60(9):1022-8. http://dx.doi.org/10.1366/000370206778397416.
Arrondo JL, Goñi FM. Infrared studies of protein-induced perturbation of lipids in lipoproteins and membranes. Chem Phys Lipids 1998;96(1-2):53-68. http://dx.doi.org/10.1016/S0009-3084(98)00080-2.
Eyring H, Jhon MS. Significant liquid structures. New York: John Wiley and Sons; 1969.
Di Paolo T, Sandorfy C. Hydrogen bond breaking potency to fluorocarbon anesthetics. J Med Chem 1974;17(8):809-14. http://dx.doi.org/10.1021/jm00254a006.
Hobza P, Mulder F, Sandorfy C. Quantum chemical and statical thermodinamic investigations of anesthetic activity. 2. The interaction between chloroform, fluoroform, and a N-H...O=C hydrogen bond. J Am Chem Soc 1982;104(4):925-8. http://dx.doi.org/10.1021/ja00368a001.
Urry DW, Sandorfy C. Chemical modulation of transmembrane protein structure and function. In: Aloia RC, Curtain CC, Gordon LM, editors. Drug and anesthetic effects on membrane structure and function. New York: Wiley-Liss; 1991. p. 91-131.
Tang P, Yan B, Xu Y. Different distribution of fluorinated anesthetics and nonanesthetics in model membrane: A 19F NMR study. Biophys J 1997;72(4):1676-82. http://dx.doi.org/10.1016/S0006-3495(97)78813-1.
Ueda I, Yoshida T. Hydration of lipid membranes and the action mechanisms of anesthetics and alcohols. Chem Phys Lipids 1999;101(1):65-79. http://dx.doi.org/10.1016/S0009-3084(99)00056-0.
Villalain J, Ortiz A, Gomez-Fernandez JC. Molecular interactions between sphingomyelin and phosphatidycholine in phospholipid vesicles. Biochim Biophys Acta 1988;941(1):55-62. http://dx.doi.org/10.1016/0005-2736(88)90213-1.
Wong PT, Mantsch HH. High pressure infrared spectroscopic evidence of water binding sites in 1,2-diacyl phospholipids. Chem Phys Lipids 1988;46(3):213-24. http://dx.doi.org/10.1016/0009-3084(88)90024-2.
Bunow MR, Levin IW. Comment on the carbon-hydrogen stretching region of vibrational Raman spectra of phospholipids. Biochim Biophys Acta 1977;487(2):388-94. http://dx.doi.org/10.1016/0005-2760(77)90015-7.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-10-04
Published 2017-02-21









