New monocyte locomotion inhibitory factor analogs protect against cerebral ischemia-reperfusion injury in rats
DOI:
https://doi.org/10.17305/bjbms.2017.1622Keywords:
Monocyte locomotion inhibitory factor, inflammatory response, neuroprotection, cerebral ischemia/reperfusion, middle cerebral artery occlusion, rat model, interleukin-1β, tumor necrosis factor-alphaAbstract
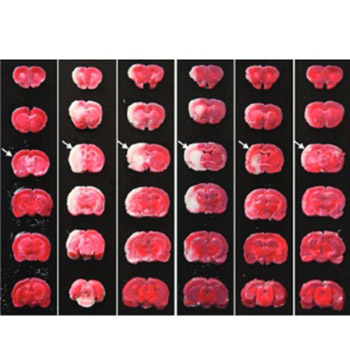
Monocyte locomotion inhibitory factor (MLIF) is an oligopeptide with anti-inflammatory properties. The carboxyl-terminal end group Cys-Asn-Ser serves as the pharmacophore of MLIF. The aim of this study was to investigate the neuroprotective effects of two new synthetic analogs, Arg-Cys-Asn-Ser and D-Cys-Asn-Ser, on focal cerebral ischemia, which were designed and synthesized to increase the penetrability and enzymatic stability of Cys-Asn-Ser. Ninety-one male Sprague-Dawley rats were randomly divided into six groups: I - Sham; II - Ischemia-reperfusion (I/R); III - Nimodipine; IV - Cys-Asn-Ser; V - D-Cys-Asn-Ser; and VI - Arg-Cys-Asn-Ser. The rats in groups II-VI were subjected to middle cerebral artery occlusion. After 24 hours of reperfusion, the neurological deficit, cerebral infarct volume, and levels of the pro-inflammatory factors interleukin-1β (IL-1β) and tumor necrosis factor-alpha in brain tissue homogenates were assessed. Compared with the sham group, the mean neurological deficit scores were significantly higher in groups II-VI (p ≤ 0.019 for all). The mean infarct volumes were significantly higher in I/R and Cys-Asn-Ser groups compared with the sham group (both p ≤ 0.046). The mean IL-1β level was significantly lower in D-Cys-Asn-Ser and Arg-Cys-Asn-Ser groups compared with I/R group (both p ≤ 0.046). In conclusion, the results showed that Arg-Cys-Asn-Ser and D-Cys-Asn-Ser have the potential for protective effects against focal cerebral ischemia injury.
Citations
Downloads
References
Dziedzic T. Systemic inflammation as a therapeutic target in acute ischemic stroke. Expert Rev Neurother 2015;15(5):523-31. https://doi.org/10.1586/14737175.2015.1035712.
Kaminska B. MAPK signalling pathways as molecular targets for anti-inflammatory therapy – From molecular mechanisms to therapeutic benefits. Biochim Biophys Acta 2005;1754(1-2):253-62. https://doi.org/10.1016/j.bbapap.2005.08.017.
Ormstad H, Verkerk R, Aass HC, Amthor KF, Sandvik L. Inflammation-induced catabolism of tryptophan and tyrosine in acute ischemic stroke. J Mol Neurosci 2013;51(3):893-902. https://doi.org/10.1007/s12031-013-0097-2.
Qiao M, Meng S, Foniok T, Tuor UI. Mild cerebral hypoxia-ischemia produces a sub-acute transient inflammatory response that is less selective and prolonged after a substantial insult. Int J Dev Neurosci 2009;27(7):691-700. https://doi.org/10.1016/j.ijdevneu.2009.07.004.
Durukan A, Tatlisumak T. Acute ischemic stroke: Overview of major experimental rodent models, pathophysiology, and therapy of focal cerebral ischemia. Pharmacol Biochem Behav 2007;87(1):179-97. https://doi.org/10.1016/j.pbb.2007.04.015.
Lin X, Yang DJ, Cai WQ, Zhao QY, Gao YF, Chen Q, et al. Endomorphins, endogenous opioid peptides, provide antioxidant defense in the brain against free radical-induced damage. Biochim Biophys Acta 2003;1639(3):195-202. https://doi.org/10.1016/j.bbadis.2003.09.007.
Ye XH, Wu Y, Guo PP, Wang J, Yuan SY, Shang Y, et al. Lipoxin A4 analogue protects brain and reduces inflammation in a rat model of focal cerebral ischemia reperfusion. Brain Res 2010;1323:174-83. https://doi.org/10.1016/j.brainres.2010.01.079.
Xin Q, Cheng B, Pan Y, Liu H, Yang C, Chen J, et al. Neuroprotective effects of apelin-13 on experimental ischemic stroke through suppression of inflammation. Peptides 2015;63:55-62. https://doi.org/10.1016/j.peptides.2014.09.016.
Xian W, Wu Y, Xiong W, Li L, Li T, Pan S, et al. The pro-resolving lipid mediator Maresin 1 protects against cerebral ischemia/reperfusion injury by attenuating the pro-inflammatory response. Biochem Biophys Res Commun 2016;472(1):175-81. https://doi.org/10.1016/j.bbrc.2016.02.090.
Kraft P, Göb E, Schuhmann MK, Göbel K, Deppermann C, Thielmann I, et al. FTY720 ameliorates acute ischemic stroke in mice by reducing thrombo-inflammation but not by direct neuroprotection. Stroke 2013;44(11):3202-10. https://doi.org/10.1161/STROKEAHA.113.002880.
Ryu S, Kwon J, Park H, Choi IY, Hwang S, Gajulapati V, et al. Amelioration of cerebral ischemic injury by a synthetic seco-nucleoside LMT497. Exp Neurobiol 2015;24(1):31-40. https://doi.org/10.5607/en.2015.24.1.31.
Hwang S, Cho GS, Ryu S, Kim HJ, Song HY, Yune TY, et al. Post-ischemic treatment of WIB801C, standardized cordyceps extract, reduces cerebral ischemic injury via inhibition of inflammatory cell migration. J Ethnopharmacol 2016;186(16):169-80. https://doi.org/10.1016/j.jep.2016.03.052.
Giménez-Scherer JA, Arenas E, Díaz L, Rico G, Fernández J, Kretschmer R. Effect of the monocyte locomotion inhibitory factor (MLIF) produced by Entamoeba histolytica on the expression of cell adhesion molecules (CAMs) in the skin of guinea pigs. Arch Med Res 2000;31(4 Suppl):S92-3. https://doi.org/10.1016/S0188-4409(00)00165-X.
Utrera-Barillas D, Velazquez JR, Enciso A, Cruz SM, Rico G, Curiel-Quesada E, et al. An anti-inflammatory oligopeptide produced by Entamoeba histolytica down-regulates the expression of pro-inflammatory chemokines. Parasite Immunol 2003;25(10):475-82. https://doi.org/10.1111/j.1365-3024.2003.00657.x.
Walsh JA. Problems in recognition and diagnosis of amebiasis: Estimation of the global magnitude of morbidity and mortality. Rev Infect Dis 1986;8(2):228-38. https://doi.org/10.1093/clinids/8.2.228.
Silva-García R, Rico-Rosillo G. Anti-inflammatory defense mechanisms of Entamoeba histolytica. Inflamm Res 2011;60(2):111-7. https://doi.org/10.1007/s00011-010-0261-x.
Kretschmer RR, Rico G, Giménez JA. A novel anti-inflammatory oligopeptide produced by Entamoeba histolytica. Mol Biochem Parasitol 2001;112(2):201-9. https://doi.org/10.1016/S0166-6851(00)00367-4.
Zhang Y, Chen J, Li F, Li D, Xiong Q, Lin Y, et al. A pentapeptide monocyte locomotion inhibitory factor protects brain ischemia injury by targeting the eEF1A1/endothelial nitric oxide synthase pathway. Stroke 2012;43(10):2764-73. https://doi.org/10.1161/STROKEAHA.112.657908.
Morales-Martínez ME, Silva-García R, Soriano-Correa C, Giménez-Scherer JA, Rojas-Dotor S, Blanco-Favela F, et al. The Cys-Asn-Ser carboxyl-terminal end group is the pharmacophore of the amebic anti-inflammatory monocyte locomotion inhibitory factor (MLIF). Mol Biochem Parasitol 2008;158(1):46-51. https://doi.org/10.1016/j.molbiopara.2007.11.010.
Barrientos-Salcedo C, Rico-Rosillo G, Giménez-Scherer JA, Soriano-Correa C. Computational study of the electronic structure characterization of a novel anti-inflammatory tripeptide derived from monocyte locomotion inhibitory factor (MLIF)-pentapeptide. Eur J Med Chem 2009;44(8):3114-9. https://doi.org/10.1016/j.ejmech.2009.03.003.
Yao J, Xu Y, Ji F, Wang C, Zhang Y, Ni J, et al. Protective effects of MLIF analogs on cerebral ischemia-reperfusion injury in rats. Peptides 2011;32(5):1047-54. https://doi.org/10.1016/j.peptides.2011.03.005.
Kretschmer R, Rico G, Giménez JA. Isolation and structural studies of the monocyte locomotion inhibitory factor (MLIF) produced by Entamoeba histolytica. Arch Med Res 2000;31(4 Suppl):S76-7. https://doi.org/10.1016/S0188-4409(00)00169-7.
Doyle KP, Simon RP, Stenzel-Poore MP. Mechanisms of ischemic brain damage. Neuropharmacology 2008;55(3):310-8. https://doi.org/10.1016/j.neuropharm.2008.01.005.
Wang Q, Tang XN, Yenari MA. The inflammatory response in stroke. J Neuroimmunol 2007;184(1-2):53-68. https://doi.org/10.1016/j.jneuroim.2006.11.014.
Wei X, Liu H, Sun X, Fu F, Zhang X, Wang J, et al. Hydroxysafflor yellow A protects rat brains against ischemia-reperfusion injury by antioxidant action. Neurosci Lett 2005;386(1):58-62. https://doi.org/10.1016/j.neulet.2005.05.069.
Kim JY, Jeong HY, Lee HK, Kim S, Hwang BY, Bae K, et al. Neuroprotection of the leaf and stem of Vitis amurensis and their active compounds against ischemic brain damage in rats and excitotoxicity in cultured neurons. Phytomedicine 2012;19(2):150-9. https://doi.org/10.1016/j.phymed.2011.06.015.
Yin L, Ohtaki H, Nakamachi T, Kudo Y, Makino R, Shioda S. Delayed expressed TNFR1 co-localize with ICAM-1 in astrocyte in mice brain after transient focal ischemia. Neurosci Lett 2004;370(1):30-5. https://doi.org/10.1016/j.neulet.2004.07.083.
Mehta SL, Manhas N, Raghubir R. Molecular targets in cerebral ischemia for developing novel therapeutics. Brain Res Rev 2007;54(1):34-66. https://doi.org/10.1016/j.brainresrev.2006.11.003.
Amantea D, Nappi G, Bernardi G, Bagetta G, Corasaniti MT. Post-ischemic brain damage: Pathophysiology and role of inflammatory mediators. FEBS J 2009;276(1):13-26. https://doi.org/10.1111/j.1742-4658.2008.06766.x.
Huang J, Upadhyay UM, Tamargo RJ. Inflammation in stroke and focal cerebral ischemia. Surg Neurol 2006;66(3):232-45. https://doi.org/10.1016/j.surneu.2005.12.028.
Gong C, Qin Z, Betz AL, Liu XH, Yang GY. Cellular localization of tumor necrosis factor alpha following focal cerebral ischemia in mice. Brain Res 1998;801(1-2):1-8. https://doi.org/10.1016/S0006-8993(98)00489-2.
Wang Q, van Hoecke M, Tang XN, Lee H, Zheng Z, Swanson RA, et al. Pyruvate protects against experimental stroke via an anti-inflammatory mechanism. Neurobiol Dis 2009;36(1):223-31. https://doi.org/10.1016/j.nbd.2009.07.018.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-10-16
Published 2017-08-20









