Human epidermal growth factor receptor 2 (HER-2) status evaluation in advanced gastric cancer using immunohistochemistry versus silver in situ hybridization
DOI:
https://doi.org/10.17305/bjbms.2016.1497Keywords:
Human epidermal growth factor receptor 2, gastric cancer, immunohistochemistry, silver in situ hybridizationAbstract
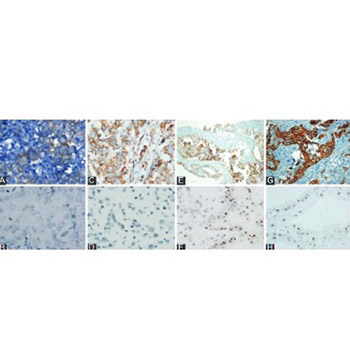
Accurate identification of human epidermal growth factor receptor 2 (HER-2) status in advanced gastric cancer patients is of utmost importance in terms of treatment planning. This study aimed to examine the HER-2 status in advanced gastric cancer patients using both immunohistochemistry (IHC) and silver in situ hybridization (SISH) techniques and to investigate concordance and diagnostic accuracy. In addition, associations between clinical parameters and HER 2 status were examined. A total of 313 patients diagnosed with locally advanced (Stage III: T3-4, N+) recurrent or metastatic adenocarcinoma of the stomach or esophagogastric junction, between 2009 and 2015, were included. HER-2 status was examined using both IHC and SISH techniques and the findings were compared. Overall SISH-confirmed HER-2 positivity rate was 22%. Multivariate analysis identified only well-differentiated tumor as a significant predictor of HER-2 positivity (OR: 2.9, 95% CI: 1.4-5.9, p = 0.003). When IHC 2+ and 3+ were considered positive for HER-2 status, sensitivity, specificity, and concordance rate (κ) was 95.7%, 93.8%, and 0.84, respectively. Corresponding figures when only IHC 3+ cases were considered positive were lower: 50%, 100%, and 0.61, respectively. The present method used for the identification of HER-2 positive gastric cancer patients provides satisfactory results. However, better categorization of IHC 2+ cases has the potential to improve the diagnostic accuracy, which is particularly important when more sophisticated methods are not readily available.
Citations
Downloads
References
. Kamangar F, Dores GM, Anderson WF. Patterns of cancer incidence, mortality, and prevalence across five continents: Defining priorities to reduce cancer disparities in different geographic regions of the world. J Clin Oncol 2006;24(14):2137-50.
http://dx.doi.org/10.1200/JCO.2005.05.2308.
. Hohenberger P, Gretschel S. Gastric cancer. Lancet 2003;362(9380):305-15. http://dx.doi.org/10.1016/S0140-6736(03)13975-X.
. Ishikawa T, Kobayashi M, Mai M, Suzuki T, Ooi A. Amplification of the c-erbB-2 (HER-2/neu) gene in gastric cancer cells. Detection by fluorescence in situ hybridization. Am J Pathol 1997;151(3):761-8.
. Ross JS, McKenna BJ. The HER-2/neu oncogene in tumors of the gastrointestinal tract. Cancer Invest 2001;19(5):554-68. http://dx.doi.org/10.1081/CNV-100103852.
. Gravalos C, Jimeno A. HER2 in gastric cancer: A new prognostic factor and a novel therapeutic target. Ann Oncol 2008;19(9):1523-9. http://dx.doi.org/10.1093/annonc/mdn169.
. Bang YJ, Van Cutsem E, Feyereislova A, Chung HC, Shen L, Sawaki A, et al. Trastuzumab in combination with chemotherapy versus chemotherapy alone for treatment of HER2-positive advanced gastric or gastro-oesophageal junction cancer (ToGA): A phase 3, open-label, randomised controlled trial. Lancet 2010;376(9742):687-97. http://dx.doi.org/10.1016/S0140-6736(10)61121-X.
. Pyo JS, Sohn JH, Kim WH. Concordance rate between HER2 immunohistochemistry and in situ hybridization in gastric carcinoma: Systematic review and meta-analysis. Int J Biol Markers 2016;31(1):e1-10.
http://dx.doi.org/10.5301/jbm.5000171.
. Hofmann M, Stoss O, Shi D, Büttner R, van de Vijver M, Kim W, et al. Assessment of a HER2 scoring system for gastric cancer: Results from a validation study. Histopathology 2008;52(7):797-805.
http://dx.doi.org/10.1111/j.1365-2559.2008.03028.x.
. Pala EE, Bayol U, Ozguzer A, Akman O. HER2 status in gastric cancer: A comparison of two novel in situ hybridization methods (IQ FISH and dual color SISH) and two immunohistochemistry methods (A0485 and HercepTest™). Pathol Res Pract 2013;209(9):548-54.
http://dx.doi.org/10.1016/j.prp.2013.05.008.
. Watson S, Validire P, Cervera P, Zorkani N, Scriva A, Lemay F, et al. Combined HER2 analysis of biopsies and surgical specimens to optimize detection of trastuzumab-eligible patients in eso-gastric adenocarcinoma: A GERCOR study. Ann Oncol 2013;24(12):3035-9. http://dx.doi.org/10.1093/annonc/mdt393.
. Park JS, Rha SY, Chung HC, Jung M, Kim KH, Jun HJ, et al. Clinicopathological features and prognostic significance of HER2 expression in gastric cancer. Oncology 2015;88(3):147-56. http://dx.doi.org/10.1159/000368555.
. Laboissiere RS, Buzelin MA, Balabram D, De Brot M, Nunes CB, Rocha RM, et al. Association between HER2 status in gastric cancer and clinicopathological features: A retrospective study using whole-tissue sections. BMC Gastroenterol 2015;15:157. http://dx.doi.org/10.1186/s12876-015-0384-1.
. Matsusaka S, Nashimoto A, Nishikawa K, Miki A, Miwa H, Yamaguchi K, et al. Clinicopathological factors associated with HER2 status in gastric cancer: Results from a prospective multicenter observational cohort study in a Japanese population (JFMC44-1101). Gastric Cancer 2016;19(3):839-51. http://dx.doi.org/10.1007/s10120-015-0518-8. http://dx.doi.org/10.1007/s10120-015-0576-y.
. Ieni A, Barresi V, Giuffrè G, Caruso RA, Lanzafame S, Villari L, et al. HER2 status in advanced gastric carcinoma: A retrospective multicentric analysis from Sicily. Oncol Lett 2013;6(6):1591-4. DOI: 10.3892/ol.2013.1611.
. Chen XZ, Zhang WH, Yao WQ, Liu JP, Zhou ZG, Chen ZX, et al. Immunohistochemical HER2 expression not associated with clinicopathological characteristics of stage I-III gastric cancer patients. Hepatogastroenterology 2014;61(134):1817-21.
. Liu X, Wang X, Wang B, Ren G, Ding W. HER2 gene amplification by fluorescence in situ hybridization (FISH) compared with immunohistochemistry (IHC) in 122 equivocal gastric cancer cases. Appl Immunohistochem Mol Morphol 2016;24(7):459-64. http://dx.doi.org/10.1097/PAI.0000000000000219.
. Cho EY, Srivastava A, Park K, Kim J, Lee MH, Do I, et al. Comparison of four immunohistochemical tests and FISH for measuring HER2 expression in gastric carcinomas. Pathology 2012;44(3):216-20. http://dx.doi.org/10.1097/PAT.0b013e3283513e8b.
. Werner D, Battmann A, Steinmetz K, Jones T, Lamb T, Martinez M, et al. The validation of a novel method combining both HER2 immunohistochemistry and HER2 dual-colour silver in situ hybridization on one slide for gastric carcinoma testing. J Transl Med 2014;12:160. http://dx.doi.org/10.1186/1479-5876-12-160.
. Koopman T, Louwen M, Hage M, Smits MM, Imholz AL. Pathologic diagnostics of HER2 positivity in gastroesophageal adenocarcinoma. Am J Clin Pathol 2015;143(2):257-64. http://dx.doi.org/10.1309/AJCPCX69HGDDGYCQ.
. Stanek L, Rozkoš T, Laco J, Ryška A, Petruželka L, Dura M, et al. Comparison of immunohistochemistry, four in situ hybridization methods and quantitative polymerase chain reaction for the molecular diagnosis of HER2 status in gastric cancer: A study of 55 cases. Mol Med Rep 2014;10(5):2669-74.
DOI: 10.3892/mmr.2014.2530.
. Park YS, Hwang HS, Park HJ, Ryu MH, Chang HM, Yook JH, et al. Comprehensive analysis of HER2 expression and gene amplification in gastric cancers using immunohistochemistry and in situ hybridization: Which scoring system should we use? Hum Pathol 2012;43(3):413-22. http://dx.doi.org/10.1016/j.humpath.2011.05.019.
. Kim MA, Jung JE, Lee HE, Yang HK, Kim WH. In situ analysis of HER2 mRNA in gastric carcinoma: Comparison with fluorescence in situ hybridization, dual-color silver in situ hybridization, and immunohistochemistry. Hum Pathol 2013;44(4):487-94. http://dx.doi.org/10.1016/j.humpath.2012.06.022.
. Chen M, Li Y, Ming Z, Biao A, Zheng LX. Comparison of HER2 status by fluorescence in situ hybridisation and immunohistochemistry in gastric cancer. Contemp Oncol (Pozn) 2014;18(2):95-9. http://dx.doi.org/10.5114/wo.2014.41383.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-08-01
Published 2017-05-20









