Significance of Foxp3+CD4+ regulatory T cells in the peripheral blood of Uygur patients in the acute and chronic phases of pigeon breeder’s lung
DOI:
https://doi.org/10.17305/bjbms.2016.1233Keywords:
Pigeon breeder’s lung, Foxp3 CD4 Treg, T lymphocyte, UygurAbstract
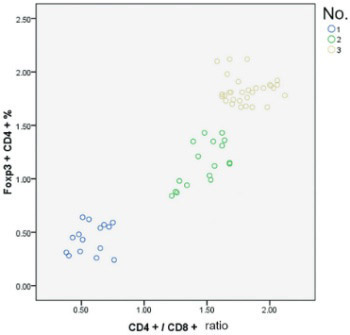
Pigeon breeder’s lung (PBL) is a type of lung inflammatory disease associated with the immune response to repeated pigeon-derived antigen exposure. The pathogenesis of PBL remains unclear. In this study, peripheral blood samples were collected from Uygur acute - and chronic-phase PBL patients and healthy subjects with pigeon contact. Foxp3+CD4+ regulatory T cell (Treg) activity in different phases of PBL was characterized by changes in Foxp3+CD4+ Treg, CD4+CD25+ T cell, and T lymphocyte subsets. Based on hypersensitivity pneumonitis (HP) diagnosis criteria, 32 PBL cases from January 2012 to December 2013 in the People’s Hospital of Xinjiang Uygur Autonomous Region Respiratory Department were included. Lung high-resolution computed tomography was performed, and the cases were classified based on the HP phase into 15 acute-phase and 17 chronic-phase cases. The control group included 30 healthy subjects with Uygur pigeon contact. Blood samples were collected, and the T cell subsets were analyzed via flow cytometry. In both PBL groups, the Foxp3+CD4+ Treg and CD4+CD25+ and CD4+CD3+ T cell percentages and CD4+/CD8+ ratios were significantly lower than in the control group (p < 0.01). In the PBL groups, particularly the acute-phase group, the CD8+CD3+ T lymphocyte percentage was significantly higher than in the control group (p < 0.01). There were no significant differences in CD4+CD25+ cells between the PBL groups. In peripheral blood from the PBL groups, the CD4+/CD8+ ratio was positively correlated with the Foxp3+CD4+ Treg (r = 0.864, p < 0.05) and CD4+/CD25+ cell (r = 0.34, p < 0.05) percentages. Low Foxp3+CD4+ Treg expression or overconsumption may be a pathogenic factor in PBL.
Citations
Downloads
References
. Barrera L, Mendoza F, Zuñiga J, Estrada A, Zamora AC, Melendro EI, et al. Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am J Respir Crit Care Med 2008;177(1):44-55.
http://dx.doi.org/10.1164/rccm.200701-093OC.
. Dalphin JC, Didier A. Environmental causes of the distal airways disease. Hypersensitivity pneumonitis and rare causes. Rev Mal Respir 2013;30(8):669-81. http://dx.doi.org/10.1016/j.rmr.2013.03.001.
. Camarena A, Aquino-Galvez A, Falfán-Valencia R, Sánchez G, Montaño M, Ramos C, et al. PSMB8 (LMP7) but not PSMB9 (LMP2) gene polymorphisms are associated to pigeon breeder's hypersensitivity pneumonitis. Respir Med 2010;104(6):889-94. http://dx.doi.org/10.1016/j.rmed.2010.01.014.
. Bourke SJ, Dalphin JC, Boyd G, McSharry C, Baldwin CI, Calvert JE. Hypersensitivity pneumonitis: Current concepts. Eur Respir J Suppl 2001;32:81s-92s.
. Girard M, Israël-Assayag E, Cormier Y. Pathogenesis of hypersensitivity pneumonitis. Curr Opin Allergy Clin Immunol 2004;4(2):93-8.
http://dx.doi.org/10.1097/00130832-200404000-00004.
. Bracken SJ, Adami AJ, Szczepanek SM, Ehsan M, Natarajan P, Guernsey LA, et al. Long-term exposure to house dust mite leads to the suppression of allergic airway disease despite persistent lung inflammation. Int Arch Allergy Immunol 2015;166(4):243-58. http://dx.doi.org/10.1159/000381058.
. Qiu SL, Bai J, Zhong XN. CD4+ Foxp3+ regulatory T cells in inflammation and emphysema after smoking cessation in rats. [Article in Chinese]. Chinese Journal of Tuberculosis and Respiratory Diseases. 2010;33(9):688-92.
. Wang LL, Ling Y, Liu S. The effect of Foxp3~+CD4~+ Treg cells in the acute and chronic greenhouse farmer’s lung. China J Mod Med 2012;22(28):40-5.
. Wang W, Wei D, Yang X. Interleukin 10 gene single nucleotide polymorphism and lung Uygur who feed pigeons. Int J Respir 2015;35(18):1401-7.
. Wu C, Chen Y, Yang X, Wang W, Pang B. Correlation of macrophage inflammatory protein-1alpha single gene polymorphisms with the susceptibility to pigeon breeder's lung in Chinese Uygur population. Int J Clin Exp Med 2015;8(8):13732-9.
. Chen JH, Juan H. Profiles of cytokines and their gene polymorphisms in the pathogenesis of pigeon breeder's lung. Curr Immunol 2004;24(3):198-202.
. Muto S, Owada Y, Inoue T, Watanabe Y, Yamaura T, Fukuhara M, et al. Clinical significance of expanded Foxp3+ Helios- regulatory T cells in patients with non-small cell lung cancer. Int J Oncol 2015;47(6):2082-90. http://dx.doi.org/10.3892/ijo.2015.3196.
. Krogulska A, Polakowska E, Wasowska-Królikowska K, Malachowska B, Mlynarski W, Borowiec M. Decreased FOXP3 mRNA expression in children with atopic asthma and IgE-mediated food allergy. Ann Allergy Asthma Immunol 2015;115(5):415-21. http://dx.doi.org/10.1016/j.anai.2015.08.015.
. Schuyler M, Cormier Y. The diagnosis of hypersensitivity pneumonitis. Chest 1997;111(3):534-6. http://dx.doi.org/10.1378/chest.111.3.534.
. Lou CY, Li M, Li L. Dynamic changes in percentages of CD4+ CD25+ regulatory T cells and Th17 cells in process of airway remodeling in mouse model of asthma. [Article in Chinese]. Chin J Contemp Pediatric 2015;17(9):994-1000.
. Maggi L, Santarlasci V, Liotta F, Frosali F, Angeli R, Cosmi L, et al. Demonstration of circulating allergen-specific CD4+CD25(high)FoxP3+ T-regulatory cells in both nonatopic and atopic individuals. J Allergy Clin Immunol 2007;120(2):429-36. http://dx.doi.org/10.1016/j.jaci.2007.05.002.
. Li S, Li Y, Qu X, Liu X, Liang J. Detection and significance of TregFoxP3+ and Th17 cells in peripheral blood of non-small cell lung cancer patients. Arch Med Sci 2014;10(2):232-9. http://dx.doi.org/10.5114/aoms.2014.42573.
. Conte E, Gili E, Fruciano M, Fagone E, Vancheri C. Human lung fibroblasts increase CD4+CD25+Foxp3+ T cells in co-cultured CD4+ lymphocytes. Cell Immunol 2013;285(1-2):55-61. http://dx.doi.org/10.1016/j.cellimm.2013.09.002.
. Chaput N, Marabelle A. Foxp3 cells are running the show in patients with surgically resected nonsmall cell lung cancer. Eur Respir J 2015;46(6):1541-3. http://dx.doi.org/10.1183/13993003.01595-2015.
. Wang LL, Zhao MJ, Mao ST. The relevance of changes of the CD4+ Foxp3+ regular T cells from different stages of elder COPD patients and the lung function. [Article in Chinese]. Chin J Gerontol 2012;32(2):223-5.
. Shi Y, Chu LL, Zhang LF. Role of CD4+CD25+ regulation cell s and expressing of FOXP3 in the pathogenesis of children with asthma. [Article in Chinese]. Acta Univ Med Nanjing 2009;29(1):117-9.
. Lee MG, Bae SC, Lee YH. Association between FOXP3 polymorphisms and susceptibility to autoimmune diseases: A meta-analysis. Autoimmunity 2015;48(7):445-52. http://dx.doi.org/10.3109/08916934.2015.1045582.
. Koschel DS, Cardoso C, Wiedemann B, Höffken G, Halank M. Pulmonary hypertension in chronic hypersensitivity pneumonitis. Lung 2012;190(3):295-302. http://dx.doi.org/10.1007/s00408-011-9361-9.
. Shi HY, Deng WJ, Zhang YP, Zhong YJ, Liu J, Fang P, et al. Expression levels of CD4+Foxp3+ regulatory T cells in the peripheral blood of patients with idiopathic pulmonary fibrosis. [Article in Chinese]. Chin J Lung Dis (Electron Ed) 2015;8(6):27-30.
. Park Y, Oh SJ, Chung DH. CD4+CD25+ regulatory T cells attenuate hypersensitivity pneumonitis by suppressing IFN-gamma production by CD4+ and CD8+ T cells. J Leukoc Biol 2009;86(6):1427-37. http://dx.doi.org/10.1189/jlb.0908542.
. Jiang H, Wu X, Zhu H, Xie Y, Tang S, Jiang Y. FOXP3+ Treg/Th17 cell imbalance in lung tissues of mice with asthma. Int J Clin Exp Med 2015;8(3):4158-63.
. Schulze B, Piehler D, Eschke M, von Buttlar H, Köhler G, Sparwasser T, et al. CD4+ FoxP3+ regulatory T cells suppress fatal T helper 2 cell immunity during pulmonary fungal infection. Eur J Immunol 2014;44(12):3596-604. http://dx.doi.org/10.1002/eji.201444963.
Downloads
Additional Files
Published
How to Cite
Accepted 2016-05-15
Published 2017-02-21









