Impact of thyroid immune-related adverse events on clinical outcomes in non-small cell lung cancer (NSCLC) patients treated with checkpoint inhibitor therapy: A single center study
DOI:
https://doi.org/10.17305/bb.2025.12321Keywords:
Non-small cell lung cancer, NSCLC, immunotherapy, immune checkpoint inhibitors, ICIs, thyroid dysfunction, immune-related adverse events, irAEs, atezolizumab, pembrolizumab, progression-free survival, PFSAbstract
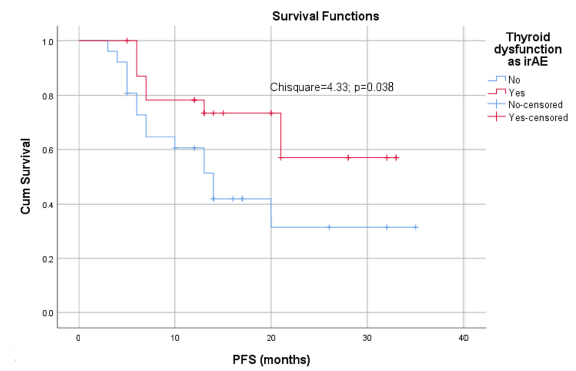
Immune checkpoint inhibitors (ICIs) have transformed the treatment landscape for non-small cell lung carcinoma (NSCLC) but are associated with immune-related adverse events (irAEs), including thyroid dysfunction. This study examines the incidence and clinical impact of thyroid dysfunction in NSCLC patients receiving ICIs at the Clinic of Oncology, Clinical Center University of Sarajevo. In this retrospective cohort study of 50 patients with metastatic NSCLC treated with ICIs—either in combination with chemotherapy or as monotherapy for those with programmed death-ligand 1 (PD-L1) expression ≥ 50%—we collected data on demographics, treatment regimens, thyroid function tests, and survival outcomes. Thyroid dysfunction occurred in 24 patients (48%), with 12 (24%) developing hypothyroidism, 4 (8%) developing hyperthyroidism, and 8 (16%) experiencing a transition from hyperthyroidism to hypothyroidism. The incidence of thyroid dysfunction was significantly higher in patients treated with atezolizumab compared to pembrolizumab (P = 0.04), with 87.5% of affected patients receiving atezolizumab. The median time to onset of thyroid dysfunction was 10 cycles (interquartile range [IQR]: 5) for hypothyroidism and six cycles (IQR: 19) for hyperthyroidism. Progression-free survival (PFS) was significantly longer in patients who developed thyroid dysfunction, with the median PFS not reached, compared to a median PFS of 14 months (95% CI: 9.68–18.32) in patients without thyroid dysfunction (P = 0.038). No significant associations were found between thyroid dysfunction and patient age or gender. These findings suggest that thyroid dysfunction is a common irAE in patients with metastatic NSCLC receiving ICIs, particularly atezolizumab, and its development may be associated with improved PFS. Regular monitoring of thyroid function is recommended to promptly identify and manage thyroid abnormalities during ICI therapy, potentially improving patient outcomes.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2025 Šejla Cerić, Timur Cerić, Emir Sokolović, Jasmina Dalač, Dragana Miletić, Inga Marijanović, Layan Mattar, Amina Aljić, Selma Agić-Bilalagić, Amera Šadija, Miran Hadžiahmetović, Semir Bešlija

This work is licensed under a Creative Commons Attribution 4.0 International License.









