TCF12 enhances angiogenesis and affects sorafenib response in liver cancer via HIF-1α interaction
DOI:
https://doi.org/10.17305/bb.2025.12022Keywords:
Transcription factor 12, TCF12, Hypoxia-Inducible Factor 1-alpha, HIF-1α, angiogenesis, sorafenibAbstract
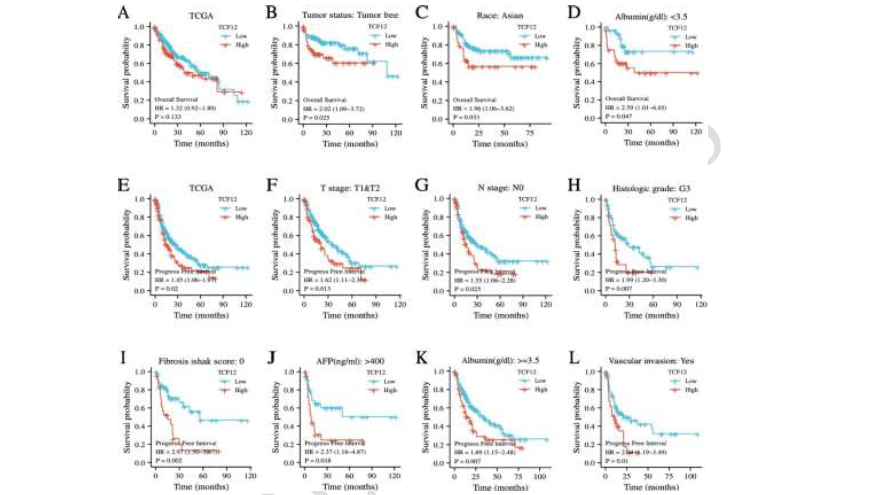
Transcription factor 12 (TCF12), a member of the basic Helix-Loop-Helix (bHLH) protein family, plays a crucial role in regulating cell growth and differentiation. It has been implicated in the development and progression of malignant tumors; however, its specific mechanisms in vascularization and drug resistance in liver cancer remain poorly understood. This study aims to explore how the interaction between TCF12 and Hypoxia-Inducible Factor 1-alpha (HIF-1α) affects vascularization and drug sensitivity in liver cancer. Using bioinformatics analysis (n = 374 TCGA samples and n = 50 clinical specimens), we assessed TCF12 expression levels in liver cancer and evaluated their association with patient prognosis. Gene Set Enrichment Analysis (GSEA) was employed to identify related signaling pathways. The expression of TCF12 in liver cancer tissues was examined via Western blotting and immunohistochemistry, while Kaplan-Meier survival analysis was used to analyze the relationship between TCF12 expression and overall survival. Functional assays—including scratch wound repair, tube formation, and endothelial cell permeability tests—were conducted to assess TCF12’s role in angiogenesis. Cell viability assays were performed to evaluate the impact of TCF12 on sorafenib sensitivity, and co-immunoprecipitation experiments were carried out to investigate the interaction between TCF12 and HIF-1α. Our bioinformatics analysis revealed that both TCF12 and HIF-1α are significantly overexpressed in liver cancer and are associated with poor prognosis. Immunohistochemical staining showed a positive correlation between TCF12 expression and the vascularization marker CD31. Furthermore, survival analysis demonstrated that patients with elevated TCF12 expression had significantly shorter overall survival. Functional assays indicated that TCF12 knockdown suppressed blood vessel formation and reduced endothelial cell permeability. Moreover, reducing TCF12 expression increased the sensitivity of liver cancer cells to sorafenib. Notably, overexpression of HIF-1α reversed these effects, and co-immunoprecipitation experiments confirmed a direct interaction between TCF12 and HIF-1α. In summary, this study demonstrates that TCF12 is highly expressed in liver cancer and is associated with poor prognosis. TCF12 promotes angiogenesis by stabilizing HIF-1α and modulates tumor sensitivity to sorafenib, highlighting its potential as a therapeutic target in liver cancer.
Citations
Downloads
Downloads
Published
Issue
Section
License
Copyright (c) 2025 Yuanbin Chen, Xiaolong Wang, Jin Chen, Min Dai, Xinyue Zhang, Jie Yin, Xiao He

This work is licensed under a Creative Commons Attribution 4.0 International License.









