Silencing CACYBP suppresses lung adenocarcinoma growth via CDK1 inhibition
DOI:
https://doi.org/10.17305/bb.2025.11849Keywords:
Calcyclin-binding protein, CACYBP, lung adenocarcinoma, LUAD, cyclin-dependent kinase 1, CDK1, PI3K/AKT pathway, proliferationAbstract
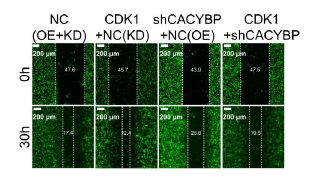
Calcyclin-binding protein (CACYBP) is a multidomain adaptor protein implicated in the development of various cancers. However, its molecular and biological roles in lung adenocarcinoma (LUAD) remain unclear. In this study, we aimed to elucidate the biological impact of CACYBP in LUAD. Immunohistochemistry was used to assess CACYBP expression in LUAD tissues. Lentivirus-mediated CACYBP knockdown was established in LUAD cell lines, and target gene expression was analyzed via Western blotting and qRT-PCR. Cell proliferation, apoptosis, and migration were evaluated using flow cytometry, colony formation assays, cell counting kit-8 (CCK 8) assays, Celigo cell counting, wound healing assays, Transwell assays, and mouse xenograft models. Co-immunoprecipitation was performed to verify the interaction between CACYBP and cyclin-dependent kinase 1 (CDK1). Additionally, the phosphoinositide 3-kinase (PI3K) inhibitor LY294002 was used to investigate the involvement of CDK1 in the PI3K/AKT pathway. Our findings revealed that CACYBP was upregulated in LUAD tissues and correlated with advanced disease stages and poor prognosis. CACYBP knockdown inhibited LUAD progression and metastasis, promoted cell apoptosis in vitro, and reduced tumorigenicity in vivo. Mechanistically, we identified CDK1 as a direct interacting partner of CACYBP. CDK1 overexpression enhanced the malignant phenotype of LUAD cells and partially reversed the inhibitory effects of CACYBP knockdown. Furthermore, inhibition of the PI3K/AKT pathway using LY294002 significantly suppressed CDK1-mediated LUAD cell growth. In conclusion, CACYBP appears to function as a tumor promoter in LUAD, at least in part through CDK1-mediated activation of the PI3K/AKT pathway. These findings suggest that CACYBP could serve as a promising therapeutic target and a novel biomarker for LUAD prognosis.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2025 Ge Wen, Shaoqing Niu, Shiqi Mei, Senming Wang

This work is licensed under a Creative Commons Attribution 4.0 International License.









