Neoadjuvant immunochemotherapy for resectable esophageal cancer: A study on efficacy and safety
DOI:
https://doi.org/10.17305/bb.2025.11806Keywords:
Immunotherapy, chemotherapy, neoadjuvant therapy, esophageal cancer, EC, neoadjuvant immunochemotherapy, nICTAbstract
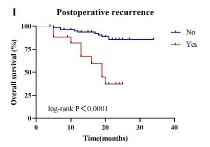
The combination of immunosuppressants and chemotherapy has reshaped the treatment landscape for esophageal cancer (EC). This study aimed to evaluate the effectiveness and safety of a neoadjuvant immunochemotherapy (nICT) regimen in patients with resectable EC. A total of 99 eligible patients were included. Data on patient characteristics, nICT regimens, surgical approaches, postoperative outcomes, adverse events (AEs) related to neoadjuvant therapy and surgery, overall survival (OS), and disease-free survival (DFS) were collected. OS, DFS, and safety were the primary endpoints. Cox regression analysis was used to identify prognostic factors in the overall population. Additionally, exploratory research was conducted to assess the clinical value of blood immune indicators in predicting tumor regression. Following surgery, 99.0% of patients achieved complete resection (R0). After neoadjuvant therapy, the number of patients with stage T0N0 increased, with complete or moderate responses being the most common outcomes according to American Joint Committee on Cancer (AJCC)/ College of American Pathologists (CAP)-tumor regression grading (TRG) evaluations (64.7%). The one-year OS and DFS rates were 91.6% and 49.3%, respectively. Grade ≥3 AEs related to neoadjuvant therapy occurred in 21.2% of patients, with gastrointestinal reactions being the most frequent (16 cases, 16.2%). No treatment-related deaths were reported. Grade ≥3 surgery-related AEs occurred in 10.1% of patients, with anastomotic leakage being the most common (six cases, 6.1%). Several factors were associated with significantly improved OS, including chemotherapy regimens combining paclitaxel with platinum, surgical approaches using laparoscopy or thoracotomy (left or right), an interval of ≤34 days between the last treatment and surgery, and the absence of positive lymph node detection. Higher cT staging was significantly associated with worse DFS. Blood immune markers, such as the neutrophil-to-lymphocyte ratio (NLR) and lymphocyte-to-monocyte ratio (LMR) were found to predict tumor regression in EC patients. In summary, nICT demonstrated favorable effectiveness and safety in resectable EC. The choice of platinum-based chemotherapy agents, rather than the type of immunosuppressant, was associated with prognosis. Moreover, a shorter interval (≤34 days) between the final nICT administration and surgery was linked to improved outcomes.
Citations
Downloads
Downloads
Additional Files
Published
Issue
Section
Categories
License
Copyright (c) 2025 Xiaomin Wang, Bingxu Li, Zhiyong Zheng, Weijie Wang

This work is licensed under a Creative Commons Attribution 4.0 International License.









