MiR-509-3p promotes gastric cancer development by activating FOXM1-mediated p38/MK2 pathway
DOI:
https://doi.org/10.17305/bb.2024.11104Keywords:
miR-509-3p, gastric cancer (GC), FOXM1, p38/MK2 pathwayAbstract
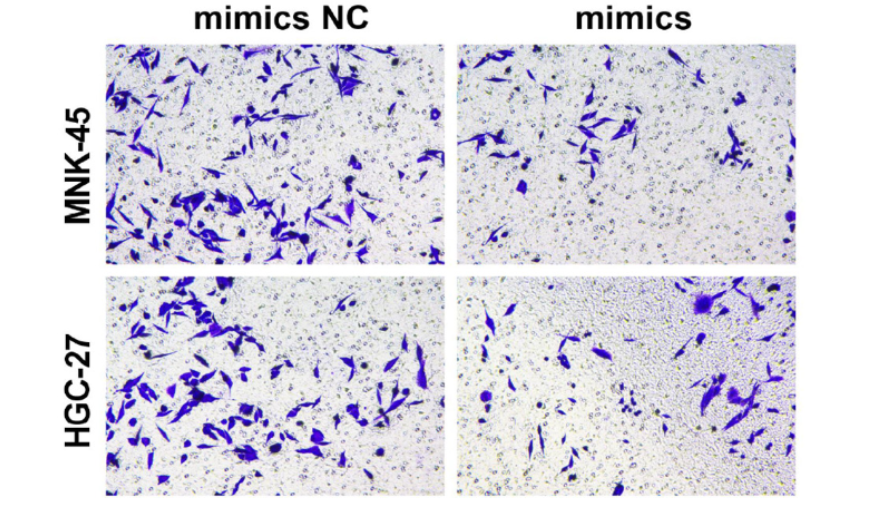
Gastric cancer (GC), a malignant tumor, is highly prevalent, particularly in Asia. miR-509-3p plays a crucial role in regulating tumorigenesis, but its mechanism in GC remains unclear. Potential targets of miR-509-3p were identified through database analyses (miRWalk, TargetScan, ENCORI, and TCGA). The binding site between miR-509-3p and forkhead box protein M1 (FOXM1) was confirmed using a dual-luciferase assay. CCK-8, EdU, Transwell, wound healing assays, flow cytometry, and Western blot analysis were employed to examine changes in proliferation, migration, invasion, apoptosis, FOXM1, and the p38 MAPK (p38)/MAPK-activated protein kinase 2 (MK2) pathway in GC cells (MNK-45 and HGC-27) after miR-509-3p overexpression or knockdown, FOXM1 overexpression, and application of the p38 pathway agonist Anisomycin. The size and weight of subcutaneous xenografts were measured, and the effects of miR-509-3p overexpression were analyzed through histopathological staining (Tunel immunofluorescence, HE staining, Ki67, and FOXM1 immunohistochemistry). The results showed that overexpression of miR-509-3p suppressed proliferation, migration, and invasion, while accelerating apoptosis. Knockdown of miR-509-3p promoted malignant progression. miR-509-3p inhibited GC by regulating FOXM1-mediated p38/MK2 pathway activation, and miR-509-3p mimics restrained tumor growth in vivo through this pathway. In conclusion, miR-509-3p suppresses GC malignant progression by regulating FOXM1-mediated p38/MK2 pathway activation.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Nan Jiang, Jiawei Kang, Yi Ding, Munire Shataer, Liangying Ma, Tayier Tuersong

This work is licensed under a Creative Commons Attribution 4.0 International License.









