Study on the mechanism of liver cancer immune escape mediated by MINDY1 through regulation of PD-L1 ubiquitination level
DOI:
https://doi.org/10.17305/bb.2024.10962Keywords:
MINDY1, PD-L1, ubiquitination, hepatocellular carcinoma, immune escape, mechanismAbstract
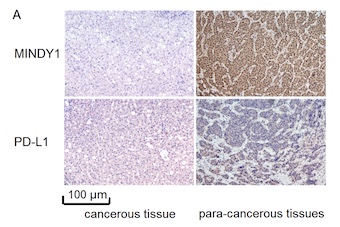
The novel deubiquitinase enzyme, motif interacting with ubiquitin-containing novel DUB family-1 (MINDY1), is highly expressed in liver cancer tissues and plays a crucial role in maintaining the stemness of liver cancer cells. Programmed death ligand-1 (PD-L1) is an immunosuppressive molecule overexpressed by tumour cells. The potential role of MINDY1 in inhibiting the stemness of liver cancer cells by deubiquitinating PD-L1 has not yet been reported. To investigate the mechanism by which MINDY1 mediates immune escape in liver cancer through the regulation of PD-L1 ubiquitination, we examined the expression levels of MINDY1 and PD-L1 in liver cancer and adjacent tissues from 50 hepatocellular carcinoma (HCC) patients using protein imprinting and immunohistochemistry. We analyzed the relationship between the expression levels of MINDY1 and PD-L1 in liver cancer tissues and their correlation with the 5-year tumor-free survival rates of patients. Subsequently, MINDY1 expression was knocked down in Huh7 cells using small interfering RNA (siRNA) interference or upregulated through transfection with a MINDY1 overexpression plasmid. The effects of MINDY1 knockdown or overexpression on the proliferation, apoptosis, migration, and invasion of HCC cells, as well as the regulation of PD-L1 binding and ubiquitination, were assessed. The 5-year tumor-free survival rates were significantly lower in both the high MINDY1 expression group and the high PD-L1 expression group (χ2 = 4.919 and 13.158, respectively). A significant difference in survival was observed between the high and low MINDY1 expression groups (χ2= 27.415). MINDY1 was found to directly interact with PD-L1, with MINDY1 gene knockdown promoting PD-L1 ubiquitination and MINDY1 overexpression inhibiting PD-L1 ubiquitination. All comparisons yielded statistically significant results (P < 0.05). In conclusion, MINDY1 inhibits the malignant progression of liver cancer by inhibiting PD-L1 ubiquitination and mediating immune escape.
Citations
Downloads
Downloads
Published
License
Copyright (c) 2024 Xingchao Song, Wenjin Li, Chunyan Tian, Xiao Ma, Weibin Yang, Jiahua Zhou

This work is licensed under a Creative Commons Attribution 4.0 International License.









