Epigenetic mechanisms of Nsd1-mediated histone methylation modifications in chondrocyte ferroptosis in knee osteoarthritis
DOI:
https://doi.org/10.17305/bb.2024.10879Keywords:
Knee osteoarthritis, ferroptosis, chondrocyte, Nsd1, Sox9, Acsl4Abstract
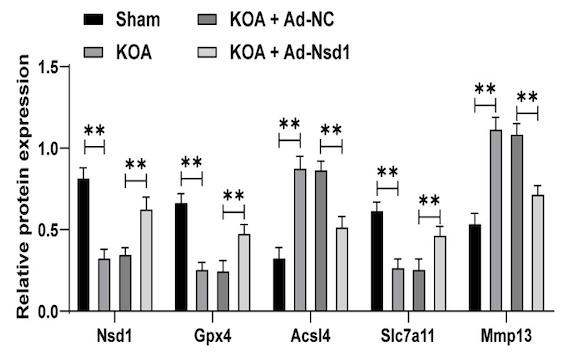
Knee osteoarthritis (KOA) is a degenerative joint disease characterized by pain, stiffness, and impaired mobility, with current therapies offering limited efficacy. This study investigates the epigenetic role of nuclear receptor-binding SET domain protein 1 (NSD1) in KOA pathogenesis. A KOA mouse model was established, and adenoviral vectors were employed to upregulate Nsd1 and inhibit SRY-box transcription factor 9 (Sox9), followed by histopathological assessments. We examined changes in cell morphology, proliferation, viability, and ferroptosis-related markers. The expression of NSD1, SOX9, and acyl-CoA synthetase long-chain family member 4 (ACSL4) was analyzed, along with the enrichment of NSD1 and dimethylated lysine 36 of histone 3 (H3K36me2) on the SOX9 promoter and SOX9 on the ACSL4 promoter. Additionally, the binding relationship between SOX9 and the ACSL4 promoter sequence was analyzed. Our results revealed that NSD1 expression was reduced in KOA mouse tissues and interleukin-1β-stimulated chondrocytes. NSD1 upregulation alleviated KOA, promoted chondrocyte proliferation and viability, and inhibited ferroptosis. Mechanistically, NSD1 enhanced H3K36me2 to upregulate SOX9 expression, which in turn suppressed ACSL4 expression and ferroptosis. SOX9 inhibition partially reversed the protective effect of NSD1 overexpression. In summary, NSD1 upregulation mitigates chondrocyte ferroptosis and ameliorates KOA by modulating H3K36me2 to upregulate SOX9 and downregulate ACSL4 expression.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Rao Wang, Da Shi, Xiaoni Pan, Anqi Ren, Kai Jiang

This work is licensed under a Creative Commons Attribution 4.0 International License.









