Salidroside exerts anti-tumor effects in ovarian cancer by inhibiting STAT3/c-Myc pathway-mediated glycolysis
DOI:
https://doi.org/10.17305/bb.2024.10867Keywords:
Ovarian cancer, salidroside, STAT3/c-Myc pathway, glycolysisAbstract
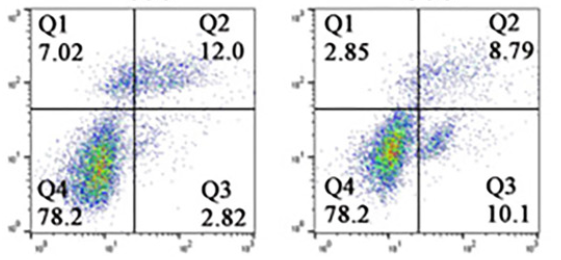
Salidroside (SAL) is a bioactive substance extracted from the traditional Chinese medicine Rhodiola rosea, which exhibits multiple pharmacological effects, such as anti-inflammatory, antioxidant, and anti-tumor properties. Currently, the effects of SAL on the malignant progression of ovarian cancer (OC) and its specific mechanism of action are not clear. Cell Counting Kit 8 (CCK-8), clone formation, Hoechst 33258 staining, flow cytometry, transwell, western blotting and immunofluorescence assays were performed to determine the impacts of SAL on the biological properties of OC cells (CAOV3 and SKOV3) and human normal ovarian epithelial cells (IOSE80). The binding activity of SAL and proteins was evaluated. Glucose consumption, lactate and ATP production, extracellular acidification rate (ECAR) and related proteins were measured to assess glycolysis. Animal models were established to evaluate the impact of SAL treatment in vivo and the expression levels of STAT3/c-Myc pathway-related proteins were determined to explore the relationship between SAL and OC. The results showed that SAL reduced the viability, clone formation, migration and invasion ability of CAOV3 and SKOV3 cells, and induced apoptosis. SAL inhibited epithelial-mesenchymal transition (EMT) and decreased glucose consumption, lactate and ATP production and ECAR. SAL exhibited good binding activity with STAT3 and c-Myc and reduced the expression levels of STAT3/c-Myc pathway and glycolysis-related proteins in vitro and in vivo. In conclusion, SAL exerted anti-tumor effects by interfering with the malignant biological progression of OC cells by inhibiting STAT3/c-Myc pathway-mediated glycolysis.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Ge Yu, Xiaoling Feng

This work is licensed under a Creative Commons Attribution 4.0 International License.









