Prognostic value of immunotherapy in advanced NSCLC based on baseline and dynamic changes in HALP
DOI:
https://doi.org/10.17305/bb.2024.10833Keywords:
NSCLC, immune checkpoint inhibitors, HALP, NLR, PLR, dynamicsAbstract
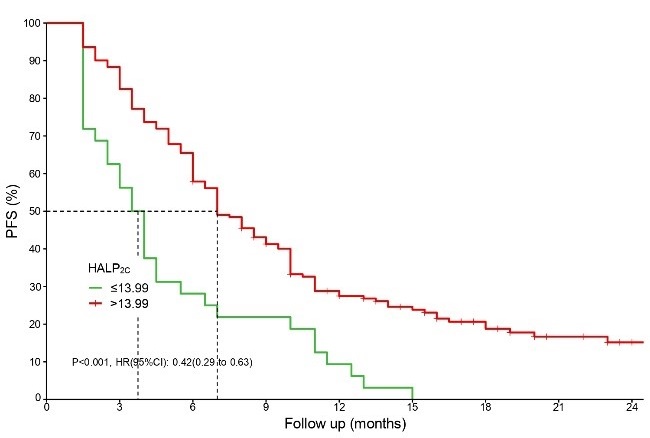
Immune checkpoint inhibitors (ICIs) enhance the tumor-killing ability of T-cells in non-small cell lung cancer (NSCLC), improving overall survival (OS) and revolutionizing treatment for advanced stages. However, challenges remain, such as low response rates and the lack of effective markers for selecting candidates. This study evaluated the impact of hemoglobin, albumin, and platelet (HALP), neutrophil-to-lymphocyte ratio (NLR), and platelet-to-lymphocyte ratio (PLR) on the efficacy of immunotherapy and survival outcomes in advanced NSCLC. Additionally, it aimed to develop a nomogram based on these parameters. Clinical and hematological data from NSCLC patients who received immunotherapy were analyzed. Efficacy was assessed using the immune Response Evaluation Criteria in Solid Tumors (iRECIST), and progression-free survival (PFS) and OS were evaluated. Prediction models incorporated baseline and post-treatment HALP, NLR, and PLR values. The 203 patients had a median follow-up of 16 months, a median PFS (mPFS) of seven months (6.0–8.0), while the median OS (mOS) was not reached (24.0–not available). Pretreatment PLR (PLR0) was associated with a higher disease control rate (DCR) (odds ratio [OR] = 0.258), while initial immunotherapy and NLR after four treatment cycles (NLR4C) significantly improved the objective response rate (ORR). Cox regression analysis showed that pretreatment HALP (HALP0), HALP after four cycles of treatment (HALP4C), and pretreatment NLR (NLR0) significantly impacted PFS. Additionally, HALP0, NLR0, and PLR after four treatment cycles (PLR4C) were associated with OS. The C-indices for PFS and OS were 0.823 and 0.878, respectively, indicating good predictive accuracy. HALP, NLR, and PLR at various time points effectively predicted immunotherapy response in advanced NSCLC patients, with low HALP combined with high NLR and PLR indicating a poor prognosis. These findings could serve as the basis for stratified randomized controlled trials (RCTs) in the future.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Hui Su, Chao Yu, Guiming Sun, Baozhong Wang, Yingjie Gao, Xiaolan Liu, Qingcui Song, Xuezhen Ma

This work is licensed under a Creative Commons Attribution 4.0 International License.









