F13B regulates angiogenesis and tumor progression in hepatocellular carcinoma via the HIF-1α/VEGF pathway
DOI:
https://doi.org/10.17305/bb.2024.10794Keywords:
F13B, HIF-1α/VEGF pathway, hepatocellular carcinoma, angiogenesis, tumor progressionAbstract
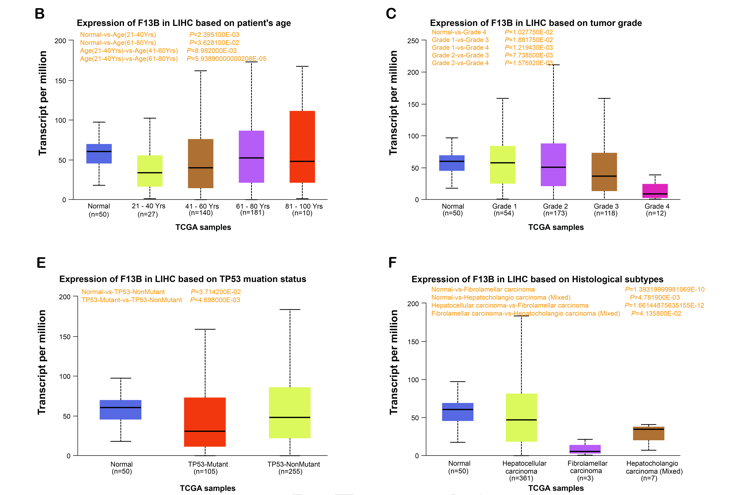
Hepatocellular carcinoma (HCC) is a highly aggressive malignant tumor with a poor prognosis. This research aimed to investigate the role of F13B in HCC and its underlying mechanisms. Through comprehensive bioinformatics analysis of the GSE120123 and The Cancer Genome Atlas (TCGA)-Liver hepatocellular carcinoma (LIHC) datasets, we identified 220 overlapping prognosis-related genes. Eight key genes, including the previously unreported CCDC170 and F13B in HCC, were identified through Least Absolute Shrinkage and Selection Operator (LASSO)-Cox regression analysis. F13B emerged as a significant prognostic factor in HCC, warranting further investigation in subsequent analyses. In vitro experiments showed that F13B expression was notably reduced in HCC cell lines and tissues, particularly in Huh-7 and SMMC-7721 cells. Overexpression of F13B inhibited cell invasion, migration, and proliferation, while its knockdown produced the opposite effect. A lactate dehydrogenase (LDH) activity assay in human umbilical vein endothelial cells (HUVECs) demonstrated that F13B overexpression reduced vascular endothelial growth factor (VEGF)-induced cytotoxicity, whereas knockdown increased it. Further analysis revealed that F13B negatively regulates VEGFA expression, affecting HUVEC proliferation. In HUVECs, F13B overexpression reversed VEGF-induced upregulation of key angiogenesis markers, including phospho-VEGF receptor 2 (p-VEGFR2), matrix metalloproteinase-2 (MMP-2), matrix metalloproteinase-9 (MMP-9), as well as AKT/mTOR signaling proteins, phospho-Akt (p-AKT), and phospho-mTOR (p-mTOR). Additionally, F13B negatively regulated VEGFA and hypoxia-inducible factor 1 A (HIF1A) under hypoxic conditions, counteracting the hypoxia-induced increase in cell viability. These findings suggest that F13B regulates angiogenesis through the HIF-1α/VEGF pathway and plays a crucial role in HCC progression. Our results highlight the potential of F13B as a therapeutic target in HCC, providing novel insights into the molecular mechanisms of HCC and its prognostic significance.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Dong Jiang, Zhi Qi, Zhi-ying Xu, Yi-ran Li

This work is licensed under a Creative Commons Attribution 4.0 International License.









