Rab8a/SNARE complex activation promotes vesicle anchoring and transport in spinal astrocytes to drive neuropathic pain
DOI:
https://doi.org/10.17305/bb.2024.10441Keywords:
Neuropathic pain, astrocytes, Rab8a, vesicular transport, SNARE proteinsAbstract
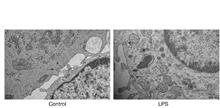
Neuropathic pain (NPP) remains a clinically challenging condition, driven by the activation of spinal astrocytes and the complex release of inflammatory mediators. This study aimed to examine the roles of Rab8a and SNARE complex proteins in activated astrocytes to uncover the underlying mechanisms of NPP. The research was conducted using a rat model with chronic constriction injury (CCI) of the sciatic nerve and primary astrocytes treated with lipopolysaccharide. Enhanced expression of Rab8a was noted specifically in spinal dorsal horn astrocytes through immunofluorescence. Electron microscopy observations showed increased vesicular transport and exocytic activity in activated astrocytes, which was corroborated by elevated levels of inflammatory cytokines such as interleukin (IL)-1β and tumor necrosis factor (TNF)-α detected through quantitative PCR. Western blot analyses confirmed significant upregulation of Rab8a, VAMP2, and Syntaxin16 in these cells. Furthermore, the application of botulinum neurotoxin type A (BONT/A) reduced the levels of vesicle transport-associated proteins, inhibiting vesicular transport in activated astrocytes. These findings suggest that the Rab8a/SNARE pathway in astrocytes enhances vesicle transport and anchoring, increasing the secretion of bioactive molecules that may play a crucial role in the pathophysiology of NPP. Inhibiting this pathway with BONT/A offers a novel therapeutic target for managing NPP, highlighting its potential utility in clinical interventions.
Citations
Downloads
Downloads
Published
Issue
Section
Categories
License
Copyright (c) 2024 Yunqiao Xiao, Gengyi Wang, Guiqiong He, Wanxiang Qin, Ying Shi

This work is licensed under a Creative Commons Attribution 4.0 International License.









