Perforation of gastric cancer - What should the surgeon do?
DOI:
https://doi.org/10.17305/bjbms.2016.1020Keywords:
Gastric cancer, surgery, perforation, survivalAbstract
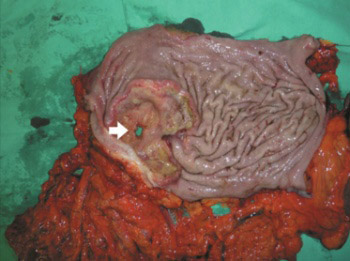
Perforation represents a rare and severe complication of gastric cancer (GC) with a large hospital mortality (8-82%). The aim of this study is to evaluate the clinical-pathological features in patients with perforated gastric cancer (PGC) and to advise the surgical treatment options. A total of 11 patients with PGC were retrospectively reviewed among 376 consecutive cases of GC operated. The clinical-pathological features including tumor stage, survival, and the type of treatment were observed. The perforation was more frequent in stage III (8 patients) and in stage IV (3 patients), but none of the cases in stage I and II GC were observed. All the patients had serosal invasion and lymph node metastasis. Limited lymphadenectomy (D0, D1) was performed in 5 patients, and extended lymphadenectomy (D2, D3) in 3 patients. Emergency gastrectomy was performed in 8 (72.8%) patients, subtotal gastrectomy in 5 (45.5%), and total gastrectomy in 3 (27.2%) cases. Three (27.2%) patients were treated by simple closure with omental patch. The overall 30-day mortality rate was 46%. The survival rate was higher among the patients who underwent curative resection (75.77±68.88 days) than in those who underwent simple closure with omental patch (18.00±24.43 days). The difference between the treatments in these groups was significant (p < 0.05). PGC required surgical emergency. Curative resection improved long-term survival in the patients with potentially curable gastric malignancy. Unsuccessful outcomes after PGC could be attributed to the poor condition of the patients and the advanced disease stage.
Citations
Downloads
References
Ozmen MM, Zulfikaroglu B, Kece C, Aslar AK, Ozalp N, Koc M. Factors influencing mortality in spontaneous gastric tumour perforations. J Int Med Res 2002;30(2):180-4. http://dx.doi.org/10.1177/147323000203000211.
Oñate-Ocaña LF, Méndez-Cruz G, Hernández-Ramos R, Becker M, Carrillo JF, Herrera-Goepfert R, et al. Experience of surgical morbidity after palliative surgery in patients with gastric carcinoma. Gastric Cancer 2007;10(4):215-20. http://dx.doi.org/10.1007/s10120-007-0437-4.
Kasakura Y, Ajani JA, Mochizuki F, Morishita Y, Fujii M, Takayama T. Outcomes after emergency surgery for gastric perforation or severe bleeding in patients with gastric cancer. J Surg Oncol 2002;80(4):181-5. http://dx.doi.org/10.1002/jso.10127.
Hata T, Sakata N, Kudoh K, Shibata C, Unno M. The best surgical approach for perforated gastric cancer: One-stage vs. two-stage gastrectomy. Gastric Cancer 2014;17(3):578-87. http://dx.doi.org/10.1007/s10120-013-0308-0.
Vasas P, Wiggins T, Chaudry A, Bryant C, Hughes FS. Emergency presentation of the gastric cancer; prognosis and implications for service planning. World J Emerg Surg 2012;7(1):31. http://dx.doi.org/10.1186/1749-7922-7-31.
Adachi Y, Aramaki M, Shiraishi N, Shimoda K, Yasuda K, Kitano S. Long-term survival after perforation of advanced gastric cancer: Case report and review of the literature. Gastric Cancer 1998;1(1):80-3. http://dx.doi.org/10.1007/s101200050059.
Kandel BP, Singh Y, Singh KP, Khakurel M. Gastric cancer perforation: Experience from a tertiary care hospital. JNMA J Nepal Med Assoc 2013;52(191):489-93.
Kotan C, Sumer A, Baser M, Kiziltan R, Carparlar MA. An analysis of 13 patients with perforated gastric carcinoma: A surgeon's nightmare? World J Emerg Surg 2008;3:17. http://dx.doi.org/10.1186/1749-7922-3-17.
Edge SB, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A. AJCC cancer staging manual. 7th ed. New York, NY: Springer; 2010.
Mahar AL, Brar SS, Coburn NG, Law C, Helyer LK. Surgical management of gastric perforation in the setting of gastric cancer. Gastric Cancer 2012;15 Suppl 1:S146-52. http://dx.doi.org/10.1007/s10120-011-0095-4.
Lehnert T, Buhl K, Dueck M, Hinz U, Herfarth C. Two-stage radical gastrectomy for perforated gastric cancer. Eur J Surg Oncol 2000;26(8):780-4. http://dx.doi.org/10.1053/ejso.2000.1003.
Ergul E, Gozetlik EO. Emergency spontaneous gastric perforations: Ulcus versus cancer. Langenbecks Arch Surg 2009;394(4):643-6.
http://dx.doi.org/10.1007/s00423-008-0331-5.
Gertsch P, Yip SK, Chow LW, Lauder IJ. Free perforation of gastric carcinoma. Results of surgical treatment. Arch Surg 1995;130(2):177-81. http://dx.doi.org/10.1001/archsurg.1995.01430020067011.
Mukai M, Kondou Y, Ogoshi K, Noto T, Makuuchi H, Tajima T. A case of perforated early gastric cancer and a review of 45 cases collected from Japanese literature. J Jpn Soc Clin Surg 1992;53:1869-73. http://dx.doi.org/10.3919/ringe1963.53.1869.
Adachi Y, Mori M, Maehara Y, Matsumata T, Okudaira Y, Sugimachi K. Surgical results of perforated gastric carcinoma: An analysis of 155 Japanese patients. Am J Gastroenterol 1997;92(3):516-8.
Tan KK, Quek TJ, Wong N, Li KK, Lim KH. Emergency surgery for perforated gastric malignancy: An institution's experience and review of the literature. J Gastrointest Oncol 2011;2(1):13-8. DOI: 10.3978/j.issn.2078-6891.2011.001
Orsenigo E, Tomajer V, Palo SD, Carlucci M, Vignali A, Tamburini A, et al. Impact of age on postoperative outcomes in 1118 gastric cancer patients undergoing surgical treatment. Gastric Cancer 2007;10(1):39-44.
http://dx.doi.org/10.1007/s10120-006-0409-0.
Jwo SC, Chien RN, Chao TC, Chen HY, Lin CY. Clinicopathological features, surgical management, and disease outcome of perforated gastric cancer. J Surg Oncol 2005;91(4):219-25. http://dx.doi.org/10.1002/jso.20307.
Viste A, Haùgstvedt T, Eide GE, Søreide O. Postoperative complications and mortality after surgery for gastric cancer. Ann Surg 1988;207(1):7-13. http://dx.doi.org/10.1097/00000658-198801000-00003.
Lee HJ, Park do J, Yang HK, Lee KU, Choe KJ. Outcome after emergency surgery in gastric cancer patients with free perforation or severe bleeding. Dig Surg 2006;23(4):217-23. http://dx.doi.org/10.1159/000094753.
Anishin NS. Perforation of gastric cancer [Article in Russian]. Vestn Khir Im I I Grek 1976;116(6):30-4.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2016-02-24
Published 2016-07-02









