CD44 silencing decreases the expression of stem cell-related factors induced by transforming growth factor β1 and tumor necrosis factor α in lung cancer: Preliminary findings
DOI:
https://doi.org/10.17305/bjbms.2017.1966Keywords:
CD44, CXCR4 receptor, Oct4, POU5F1, non-small cell lung cancer cells, NSCLC, C-X-C chemokine receptor type 4, octamer-binding transcription factor 4Abstract
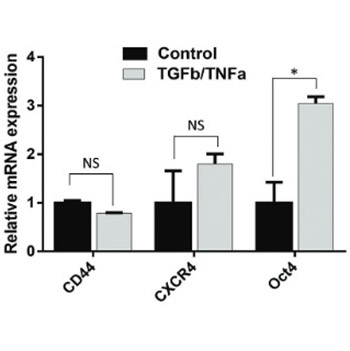
The mechanism underlying increased concentrations of cancer stem cell (CSC)-associated factors in non-small cell lung cancer (NSCLC) cells treated with transforming growth factor β1 (TGFβ1) and tumor necrosis factor α (TNFα), is still not clear. The purpose of this study was to investigate the possible role of CD44 in the regulation of CSC-associated genes, by analyzing the effect of CD44 knockdown on their expression. A549, a NSCLC cell line that expresses CD44 antigen, was treated with TGFβ1 and TNFα. Small-interfering ribonucleic acid (siRNA) that specifically targets the CD44 gene was used to knockdown the expression of CD44 in A549. The gene expressions of CD44, CXCR4, POU5F1 (octamer-binding transcription factor 4 [Oct4]), PROM1, NANOG, c-Myc, KLF4, and SOX2, as well as of CDH1 (E-cadherin), CDH2 (N-cadherin), VIM (vimentin), and FN1 (fibronectin) were analyzed in A549 cells by quantitative reverse transcription polymerase chain reaction (RT-qPCR). Cell morphology was observed using light microscopy. After TGFβ1/TNFα treatment, increased expressions of CXCR4 and POU5F1 were detected. Silencing of CD44 gene expression was confirmed by RT-qPCR. The knockdown of CD44 decreased the CXCR4 and POU5F1 gene expressions in TGFβ1/TNFα-treated A549 cells. However, the silencing of CD44 did not affect the morphology of TGFβ1/TNFα-treated A549 cells nor it reversed epithelial-mesenchymal transition (EMT) gene signature induced by TGFβ1/TNFα in A549 cells. Our preliminary findings suggest that the CD44 gene may have a role in regulating CXCR4 and POU5F1 gene expressions, independently of the EMT signaling pathway.
Citations
Downloads
References
Al-Hajj M, Becker MW, Wicha M, Weissman I, Clarke MF. Therapeutic implications of cancer stem cells. Curr Opin Genet Dev 2004;14(1):43-7. https://doi.org/10.1016/j.gde.2003.11.007.
Murakami A, Takahashi F, Nurwidya F, Kobayashi I, Minakata K, Hashimoto M, et al. Hypoxia increases gefitinib-resistant lung cancer stem cells through the activation of insulin-like growth factor 1 receptor. PLoS One 2014;9(1):e86459. https://doi.org/10.1371/journal.pone.0086459.
Nurwidya F, Murakami A, Takahashi F, Takahashi K. Lung cancer stem cells: Tumor biology and clinical implications. Asia Pac J Clin Oncol 2012;8(3):217-22. https://doi.org/10.1111/j.1743-7563.2012.01550.x.
Fillmore C, Kuperwasser C. Human breast cancer stem cell markers CD44 and CD24: Enriching for cells with functional properties in mice or in man? Breast Cancer Res 2007;9(3):303. https://doi.org/10.1186/bcr1673.
de Beca FF, Caetano P, Gerhard R, Alvarenga CA, Gomes M, Paredes J, et al. Cancer stem cells markers CD44, CD24 and ALDH1 in breast cancer special histological types. J Clin Pathol 2013;66(3):187-91. https://doi.org/10.1136/jclinpath-2012-201169.
Al-Hajj M, Wicha MS, Benito-Hernandez A, Morrison SJ, Clarke MF. Prospective identification of tumorigenic breast cancer cells. Proc Natl Acad Sci U S A 2003;100(7):3983-8. https://doi.org/10.1073/pnas.0530291100.
Jung MJ, Rho JK, Kim YM, Jung JE, Jin YB, Ko YG, et al. Upregulation of CXCR4 is functionally crucial for maintenance of stemness in drug-resistant non-small cell lung cancer cells. Oncogene 2013;32(2):209-21. https://doi.org/10.1038/onc.2012.37.
Kim RJ, Nam JS. Oct4 expression enhances features of cancer stem cells in a mouse model of breast cancer. Lab Anim Res 2011;27(2):147-52. https://doi.org/10.5625/lar.2011.27.2.147.
Leung EL, Fiscus RR, Tung JW, Tin VP, Cheng LC, Sihoe AD, et al. Non-small cell lung cancer cells expressing CD44 are enriched for stem cell-like properties. PLoS One 2010;5(11):e14062. https://doi.org/10.1371/journal.pone.0014062.
Ohashi R, Takahashi F, Cui R, Yoshioka M, Gu T, Sasaki S, et al. Interaction between CD44 and hyaluronate induces chemoresistance in non-small cell lung cancer cell. Cancer Lett 2007;252(2):225-34. https://doi.org/10.1016/j.canlet.2006.12.025.
Takahashi K, Takahashi F, Hirama M, Tanabe KK, Fukuchi Y. Restoration of CD44S in non-small cell lung cancer cells enhanced their susceptibility to the macrophage cytotoxicity. Lung Cancer 2003;41(2):145-53.
https://doi.org/10.1016/S0169-5002(03)00224-1.
Pirozzi G, Tirino V, Camerlingo R, Franco R, La Rocca A, Liguori E, et al. Epithelial to mesenchymal transition by TGFβ-1 induction increases stemness characteristics in primary non small cell lung cancer cell line. PLoS One 2011;6(6):e21548. https://doi.org/10.1371/journal.pone.0021548.
Cufi S, Vazquez-Martin A, Oliveras-Ferraros C, Martin-Castillo B, Joven J, Menendez JA. Metformin against TGFbeta-induced epithelial-to-mesenchymal transition (EMT): From cancer stem cells to aging-associated fibrosis. Cell Cycle 2010;9(22):4461-8. https://doi.org/10.4161/cc.9.22.14048.
Yamasaki S, Taguchi Y, Shimamoto A, Mukasa H, Tahara H, Okamoto T. Generation of human induced pluripotent stem (Ips) cells in serum- and feeder-free defined culture and TGF-B1 regulation of pluripotency. PLoS One 2014;9(1):e87151. https://doi.org/10.1371/journal.pone.0087151.
Mima K, Okabe H, Ishimoto T, Hayashi H, Nakagawa S, Kuroki H, et al. CD44s regulates the TGF-β-mediated mesenchymal phenotype and is associated with poor prognosis in patients with hepatocellular carcinoma. Cancer Res 2012;72(13):3414-23. https://doi.org/10.1158/0008-5472.CAN-12-0299.
Chen S, Tuttle DL, Oshier JT, Knot HJ, Streit WJ, Goodenow MM, et al. Transforming growth factor-beta1 increases CXCR4 expression, stromal-derived factor-1alpha-stimulated signalling and human immunodeficiency virus-1 entry in human monocyte-derived macrophages. Immunology 2005;114(4):565-74. https://doi.org/10.1111/j.1365-2567.2004.02110.x.
Chu CY, Sheen YS, Cha ST, Hu YF, Tan CT, Chiu HC, et al. Induction of chemokine receptor CXCR4 expression by transforming growth factor-β1 in human basal cell carcinoma cells. J Dermatol Sci 2013;72(2):123-33. https://doi.org/10.1016/j.jdermsci.2013.06.011.
Tirino V, Camerlingo R, Bifulco K, Irollo E, Montella R, Paino F, et al. TGF-β1 exposure induces epithelial to mesenchymal transition both in CSCs and non-CSCs of the A549 cell line, leading to an increase of migration ability in the CD133+ A549 cell fraction. Cell Death Dis 2013;4:e620. https://doi.org/10.1038/cddis.2013.144.
Naor D, Sionov RV, Ish-Shalom D. CD44: Structure, function, and association with the malignant process. Adv Cancer Res 1997;71:241-319.
https://doi.org/10.1016/S0065-230X(08)60101-3.
Eliaz RE, Szoka FC Jr. Liposome-encapsulated doxorubicin targeted to CD44: A strategy to kill CD44-overexpressing tumor cells. Cancer Res 2001;61(6):2592-601.
Orian-Rousseau V, Ponta H. Perspectives of CD44 targeting therapies. Arch Toxicol 2015;89(1):3-14. DOI: 10.1007/s00204-014-1424-2.
Arpicco S, De Rosa G, Fattal E. Lipid-based nanovectors for targeting of CD44-overexpressing tumor cells. J Drug Deliv 2013;2013:860780. https://doi.org/10.1155/2013/860780.
Shah V, Taratula O, Garbuzenko OB, Taratula OR, Rodriguez-Rodriguez L, Minko T. Targeted nanomedicine for suppression of CD44 and simultaneous cell death induction in ovarian cancer: An optimal delivery of siRNA and anticancer drug. Clin Cancer Res 2013;19(22):6193-204. https://doi.org/10.1158/1078-0432.CCR-13-1536.
Asai-Tajiri Y, Matsumoto K, Fukuyama S, Kan OK, Nakano T, Tonai K, et al. Small interfering RNA against CD86 during allergen challenge blocks experimental allergic asthma. Respir Res 2014;15:132. https://doi.org/10.1186/s12931-014-0132-z.
Fuchs K, Hippe A, Schmaus A, Homey B, Sleeman JP, Orian-Rousseau V. Opposing effects of high- and low-molecular weight hyaluronan on CXCL12-induced CXCR4 signaling depend on CD44. Cell Death Dis 2013;4:e819. https://doi.org/10.1038/cddis.2013.364.
Downloads
Additional Files
Published
Issue
Section
Categories
How to Cite
Accepted 2017-02-25
Published 2017-08-20









