Tocilizumab inhibits neuronal cell apoptosis and activates STAT3 in cerebral infarction rat model
DOI:
https://doi.org/10.17305/bjbms.2016.853Keywords:
Tocilizumab, cerebral infarction, apoptosis, STAT3, interleukin 6Abstract
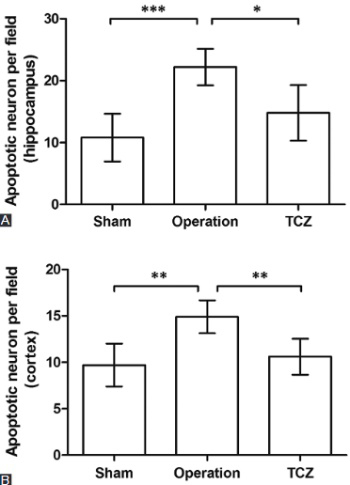
Cerebral infarction is a severe hypoxic ischemic necrosis with accelerated neuronal cell apoptosis in the brain. As a monoclonal antibody against interleukin 6, tocilizumab (TCZ) is widely used in immune diseases, whose function in cerebral infarction has not been studied. This study aims to reveal the role of TCZ in regulating neuronal cell apoptosis in cerebral infarction. The cerebral infarction rat model was constructed by middle cerebral artery occlusion and treated with TCZ. Cell apoptosis in hippocampus and cortex of the brain was examined with TUNEL method. Rat neuronal cells cultured in oxygen-glucose deprivation (OGD) conditions and treated with TCZ were used to compare cell viability and apoptosis. Apoptosis-related factors including B-cell lymphoma extra large (Bcl-xL) and Caspase 3, as well as the phosphorylated signal transducer and activator of transcription 3 (p-STAT3) in brain cortex were analyzed from the protein level. Results indicated that TCZ treatment could significantly prevent the promoted cell apoptosis caused by cerebral infarction or OGD (P < 0.05 or P < 0.01). In brain cortex of the rat model, TCZ up-regulated Bcl-xL and down-regulated Caspase 3, consistent with the inhibited cell apoptosis. It also promoted tyrosine 705 phosphorylation of STAT3, which might be the potential regulatory mechanism of TCZ in neuronal cells. This study provided evidence for the protective role of TCZ against neuronal cell apoptosis in cerebral infarction. Based on these fundamental data, TCZ is a promising option for treating cerebral infarction, but further investigations on related mechanisms are still necessary.
Downloads
References
Gjerde G, Naess H. Risk factor burden predicts long-term mortality after cerebral infarction. Acta Neurol Scand 2014;129(3):173-177.
http://dx.doi.org/10.1111/ane.12159
Fugate JE, Rabinstein AA. Contraindications to intravenous rtPA for acute stroke: A critical reappraisal. Neurology Clinical Practice 2013;3(3):177-185.
http://dx.doi.org/10.1212/CPJ.0b013e318296f0a9
Jeong HS, Song HJ, Kim SB, Lee J, Kang CW, Koh HS, et al. A comparison of stent-assisted mechanical thrombectomy and conventional intra-arterial thrombolysis for acute cerebral infarction. J Clin Neurol 2013;9(2):91-96.
http://dx.doi.org/10.3988/jcn.2013.9.2.91
Han KT, Park EC, Kim SJ, Kim W, Hahm MI, Jang SI, et al. Effective strategy for improving health care outcomes: Multidisciplinary care in cerebral infarction patients. Health Policy 2015;119(8):1039-1045.
http://dx.doi.org/10.1016/j.healthpol.2015.06.005
Ling L, Zeng J, Pei Z, Cheung RT, Hou Q, Xing S, et al. Neurogenesis and angiogenesis within the ipsilateral thalamus with secondary damage after focal cortical infarction in hypertensive rats. J Cereb Blood Flow Metab 2009;29(9):1538-1546.
http://dx.doi.org/10.1038/jcbfm.2009.76
Liao SJ, Gong Q, Chen XR, Ye LX, Ding Q, Zeng JS, et al. Netrin-1 rescues neuron loss by attenuating secondary apoptosis in ipsilateral thalamic nucleus following focal cerebral infarction in hypertensive rats. Neuroscience 2013;231(225-232.
Matsushita K, Iwanaga S, Oda T, Kimura K, Shimada M, Sano M, et al. Interleukin-6/soluble interleukin-6 receptor complex reduces infarct size via inhibiting myocardial apoptosis. Lab Invest 2005;85(10):1210-1223.
http://dx.doi.org/10.1038/labinvest.3700322
Oritani K, Tomiyama Y, Kincade PW, Aoyama K, Yokota T, Matsumura I, et al. Both stat3-activation and stat3-independent BCL2 downregulation are important for interleukin-6-induced apoptosis of 1A9-M cells. Blood 1999;93(4):1346-1354.
Minichsdorfer C, Wasinger C, Sieczkowski E, Atil B, Hohenegger M. Tocilizumab unmasks a stage-dependent interleukin-6 component in statin-induced apoptosis of metastatic melanoma cells. Melanoma Res 2015;25(4):284-294.
http://dx.doi.org/10.1097/CMR.0000000000000172
Lin JZ, M KQ, Zhang HX, Kong QZ, Yuan RM, Wang ZW, et al. Change of early serum TNF-alpha and IL-6 levels in acute cerebral infarction and its significances. Journal of Zhejiang University (Medical Sciences) 2010;39(4):415-418.
Donghong L, Jinying C, Ning P, Zheng W, Xiangdong Z. The clinical significance of the expressions of hs-CRP, Hcy and IL-6 in patients with acute cerebral infarction. China Modern Doctor 2014;52(11):27-30.
Suzuki M, Hashizume M, Yoshida H, Mihara M. Anti-inflammatory mechanism of tocilizumab, a humanized anti-IL-6R antibody: effect on the expression of chemokine and adhesion molecule. Rheumatol Int 2010;30(3):309-315.
http://dx.doi.org/10.1007/s00296-009-0953-0
Smolen JS, Beaulieu A, Rubbert-Roth A, Ramos-Remus C, Rovensky J, Alecock E, et al. Effect of interleukin-6 receptor inhibition with tocilizumab in patients with rheumatoid arthritis (OPTION study): a double-blind, placebo-controlled, randomised trial. The Lancet 2008;371(9617):987-997.
http://dx.doi.org/10.1016/S0140-6736(08)60453-5
Hardwick JM, Soane L. Multiple functions of BCL-2 family proteins. Cold Spring Harb Perspect Biol 2013;5(2):doi: 10.1101/cshperspect.a008722.
http://dx.doi.org/10.1101/cshperspect.a008722
Willimott S, Merriam T, Wagner SD. Apoptosis induces Bcl-XS and cleaved Bcl-XL in chronic lymphocytic leukaemia. Biochem Biophys Res Commun 2011;405(3):480-485.
http://dx.doi.org/10.1016/j.bbrc.2011.01.057
Mishra DP, Pal R, Shaha C. Changes in cytosolic Ca2+ levels regulate Bcl-xS and Bcl-xL expression in spermatogenic cells during apoptotic death. J Biol Chem 2006;281(4):2133-2143.
http://dx.doi.org/10.1074/jbc.M508648200
Ji F, Zhang H, Wang Y, Li M, Xu W, Kang Y, et al. MicroRNA-133a, downregulated in osteosarcoma, suppresses proliferation and promotes apoptosis by targeting Bcl-xL and Mcl-1. Bone 2013;56(1):220-226.
http://dx.doi.org/10.1016/j.bone.2013.05.020
Del Re DP, Matsuda T, Zhai P, Maejima Y, Jain MR, Liu T, et al. Mst1 promotes cardiac myocyte apoptosis through phosphorylation and inhibition of Bcl-xL. Mol Cell 2014;54(4):639-650.
http://dx.doi.org/10.1016/j.molcel.2014.04.007
Brentnall M, Rodriguez-Barrueco R, De Guevara RL, Cepero E, Boise LH. Caspase-9, caspase-3 and caspase-7 have distinct roles during intrinsic apoptosis. BMC Cell Biology 2013;14(32):doi: 10.1186/1471-2121-1114-1132.
Kim JM, Luo L, Zirkin BR. Caspase-3 activation is required for leydig cell apoptosis induced by ethane dimethanesulfonate. Endocrinology 2000;141(5):1846-1853.
http://dx.doi.org/10.1210/en.141.5.1846
Liu CY, Su JC, Ni MH, Tseng LM, Chu PY, Wang DS, et al. Obatoclax analog SC-2001 inhibits STAT3 phosphorylation through enhancing SHP-1 expression and induces apoptosis in human breast cancer cells. Breast Cancer Res Treat 2014;146(1):71-84.
http://dx.doi.org/10.1007/s10549-014-3000-0
Gupta SC, Phromnoi K, Aggarwal BB. Morin inhibits STAT3 tyrosine 705 phosphorylation in tumor cells through activation of protein tyrosine phosphatase SHP1. Biochem Pharmacol 2013;85(7):898-912.
http://dx.doi.org/10.1016/j.bcp.2012.12.018
Lee TL, Yeh J, Friedman J, Yan B, Yang X, Yeh NT, et al. A signal network involving coactivated NF-kappaB and STAT3 and altered p53 modulates BAX/BCL-XL expression and promotes cell survival of head and neck squamous cell carcinomas. Int J Cancer 2008;122(9):1987-1998.
http://dx.doi.org/10.1002/ijc.23324
Hodge DR, Hurt EM, Farrar WL. The role of IL-6 and STAT3 in inflammation and cancer. Eur J Cancer 2005;41(16):2502-2512.
http://dx.doi.org/10.1016/j.ejca.2005.08.016
Yeh HH, Lai WW, Chen HH, Liu HS, Su WC. Autocrine IL-6-induced Stat3 activation contributes to the pathogenesis of lung adenocarcinoma and malignant pleural effusion. Oncogene 2006;25(31):4300-4309.
http://dx.doi.org/10.1038/sj.onc.1209464
Lin J, Tang H, Jin X, Jia G, Hsieh JT. p53 regulates Stat3 phosphorylation and DNA binding activity in human prostate cancer cells expressing constitutively active Stat3. Oncogene 2002;21(19):3082-3088.
http://dx.doi.org/10.1038/sj.onc.1205426
Wery-Zennaro S, Letourneur M, David M, Bertoglio J, Pierre J. Binding of IL-4 to the IL-13Rα1/IL-4Rα receptor complex leads to STAT3 phosphorylation but not to its nuclear translocation. FEBS Lett 1999;464(1-2):91-96.
http://dx.doi.org/10.1016/S0014-5793(99)01680-4
Kim NH, Lee MY, Park SJ, Choi JS, Oh MK, Kim IS. Auranofin blocks interleukin-6 signalling by inhibiting phosphorylation of JAK1 and STAT3. Immunology 2007;122(4):607-614.
http://dx.doi.org/10.1111/j.1365-2567.2007.02679.x
Feng Q, Wang YI, Yang Y. Neuroprotective effect of interleukin-6 in a rat model of cerebral ischemia. Exp Ther Med 2015;9(5):1695-1701.
http://dx.doi.org/10.3892/etm.2015.2363
Hiura M, Abe S, Tabaru A, Shimajiri S, Hanami K, Saito K, et al. Case of severe liver damage after the induction of tocilizumab therapy for rheumatoid vasculitis. Hepatol Res 2011;41(5):492-496.