Modulation of iron metabolism by iron chelation regulates intracellular calcium and increases sensitivity to doxorubicin
DOI:
https://doi.org/10.17305/bjbms.2016.576Keywords:
Intracellular calcium, iron uptake, doxorubicin, desferrioxamineAbstract
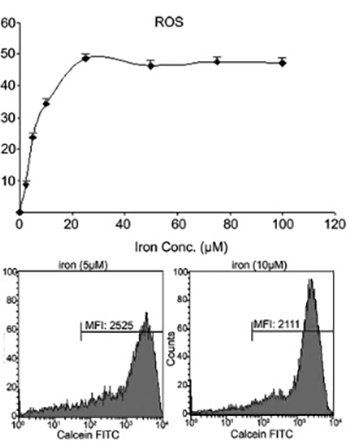
Increased intracellular iron levels can both promote cell proliferation and death, as such; iron has a “two-sided effect” in the delicate balance of human health. Though the role of iron in the development of cancer remains unclear, investigations of iron chelators as anti-tumor agents have revealed promising results. Here, we investigated the influence of iron and desferrioxamine (DFO), the iron chelating agent on intracellular calcium in a human leukemia cell line, K562. Iron uptake is associated with increased reactive oxygen species (ROS) generation. Therefore, we showed that iron also caused dose-dependent ROS generation in K562 cells. The measurement of intracellular calcium was determined using Furo-2 with a fluorescence spectrophotometer. The iron delivery process to the cytoplasmic iron pool was examined by monitoring the fluorescence of cells loaded with calcein-acetoxymethyl. Our data showed that iron increased intracellular calcium, and this response was 8 times higher when cells were incubated with DFO. K562 cells with DFO caused a 3.5 times increase of intracellular calcium in the presence of doxorubicin (DOX). In conclusion, DFO induces intracellular calcium and increases their sensitivity to DOX, a chemotherapeutic agent.
Downloads
References
Orrenius S, Zhivotovsky B, Nicotera P. Regulation of cell death: The calcium-apoptosis link. Nat Rev Mol Cell Biol 2003;4(7):552-65. http://dx.doi.org/10.1038/nrm1150
Cazzola M, Bergamaschi G, Dezza L, Arosio P. Manipulations of cellular iron metabolism for modulating normal and malignant cell proliferation: Achievements and prospects. Blood 1990;75(10):1903-19.
Hoffbrand AV, Ganeshaguru K, Hooton JW, Tattersall MH. Effect of iron deficiency and desferrioxamine on DNA synthesis in human cells. Br J Haematol 1976;33(4):517-26. http://dx.doi.org/10.1111/j.1365-2141.1976.tb03570.x.
Pantopoulos K, Porwal SK, Tartakoff A, Devireddy L. Mechanisms of mammalian iron homeostasis. Biochemistry 2012;51(29):5705-24. http://dx.doi.org/10.1021/bi300752r.
Pogribny IP, Tryndyak VP, Pogribna M, Shpyleva S, Surratt G, Gamboa da Costa G, et al. Modulation of intracellular iron metabolism by iron chelation affects chromatin remodeling proteins and corresponding epigenetic modifications in breast cancer cells and increases their sensitivity to chemotherapeutic agents. Int J Oncol 2013;42(5):1822-32. http://dx.doi.org/10.3892/ijo.2013.1855.
Torti SV, Torti FM. Ironing out cancer. Cancer Res 2011;71(5):1511-4. http://dx.doi.org/10.1158/0008-5472.CAN-10-3614.
Jones PA, Baylin SB. The epigenomics of cancer. Cell 2007;128(4):683-92. http://dx.doi.org/10.1016/j.cell.2007.01.029.
Torti SV, Torti FM. Iron and cancer: More ore to be mined. Nat Rev Cancer 2013;13(5):342-55. http://dx.doi.org/10.1038/nrc3495.
Buss JL, Torti FM, Torti SV. The role of iron chelation in cancer therapy. Curr Med Chem 2003;10(12):1021-34. http://dx.doi.org/10.2174/0929867033457638.
Yu Y, Gutierrez E, Kovacevic Z, Saletta F, Obeidy P, Suryo Rahmanto Y, et al. Iron chelators for the treatment of cancer. Curr Med Chem 2012;19(17):2689-702. http://dx.doi.org/10.2174/092986712800609706.
Rao VA, Klein SR, Agama KK, Toyoda E, Adachi N, Pommier Y, et al. The iron chelator Dp44mT causes DNA damage and selective inhibition of topoisomerase IIalpha in breast cancer cells. Cancer Res 2009;69(3):948-57. http://dx.doi.org/10.1158/0008-5472.CAN-08-1437.
Günes DA, Florea AM, Splettstoesser F, Büsselberg D. Co-application of arsenic trioxide (As2O3) and cisplatin (CDDP) on human SY-5Y neuroblastoma cells has differential effects on the intracellular calcium concentration ([Ca2 ]i) and cytotoxicity. Neurotoxicology 2009;30(2):194-202. http://dx.doi.org/10.1016/j.neuro.2008.12.001.
Tenopoulou M, Kurz T, Doulias PT, Galaris D, Brunk UT. Does the calcein-AM method assay the total cellular 'labile iron pool' or only a fraction of it? Biochem J 2007;403(2):261-6. http://dx.doi.org/10.1042/BJ20061840.
Bindokas VP, Jordán J, Lee CC, Miller RJ. Superoxide production in rat hippocampal neurons: Selective imaging with hydroethidine. J Neurosci 1996;16(4):1324-36.
Li N, Ragheb K, Lawler G, Sturgis J, Rajwa B, Melendez JA, et al. Mitochondrial complex I inhibitor rotenone induces apoptosis through enhancing mitochondrial reactive oxygen species production. J Biol Chem 2003;278(10):8516-25. http://dx.doi.org/10.1074/jbc.M210432200.
Grynkiewicz G, Poenie M, Tsien RY. A new generation of Ca2+ indicators with greatly improved fluorescence properties. J Biol Chem 1985;260(6):3440-50.
Hoke EM, Maylock CA, Shacter E. Desferal inhibits breast tumor growth and does not interfere with the tumoricidal activity of doxorubicin. Free Radic Biol Med 2005;39(3):403-11. http://dx.doi.org/10.1016/j.freeradbiomed.2005.03.029.
Whitnall M, Howard J, Ponka P, Richardson DR. A class of iron chelators with a wide spectrum of potent antitumor activity that overcomes resistance to chemotherapeutics. Proc Natl Acad Sci U S A 2006;103(40):14901-6. http://dx.doi.org/10.1073/pnas.0604979103.
Pinnix ZK, Miller LD, Wang W, D'Agostino R Jr, Kute T, Willingham MC, et al. Ferroportin and iron regulation in breast cancer progression and prognosis. Sci Transl Med 2010;2(43):43ra56. http://dx.doi.org/10.1126/scitranslmed.3001127.
Le NT, Richardson DR. The role of iron in cell cycle progression and the proliferation of neoplastic cells. Biochim Biophys Acta 2002;1603(1):31-46. http://dx.doi.org/10.1016/s0304-419x(02)00068-9.
Wilkinson MG, Millar JB. Control of the eukaryotic cell cycle by MAP kinase signaling pathways. FASEB J 2000;14(14):2147-57. http://dx.doi.org/10.1096/fj.00-0102rev.
Saletta F, Suryo Rahmanto Y, Noulsri E, Richardson DR. Iron chelator-mediated alterations in gene expression: Identification of novel iron-regulated molecules that are molecular targets of hypoxia-inducible factor-1 alpha and p53. Mol Pharmacol 2010;77(3):443-58. http://dx.doi.org/10.1124/mol.109.061028.
Le NT, Richardson DR. Iron chelators with high antiproliferative activity up-regulate the expression of a growth inhibitory and metastasis suppressor gene: A link between iron metabolism and proliferation. Blood 2004;104(9):2967-75. http://dx.doi.org/10.1182/blood-2004-05-1866.
Hileti D, Panayiotidis P, Hoffbrand AV. Iron chelators induce apoptosis in proliferating cells. Br J Haematol 1995;89(1):181-7. http://dx.doi.org/10.1111/j.1365-2141.1995.tb08927.x.
Pan YJ, Hopkins RG, Loo G. Increased GADD153 gene expression during iron chelation-induced apoptosis in Jurkat T-lymphocytes. Biochim Biophys Acta 2004;1691(1):41-50. http://dx.doi.org/10.1016/j.bbamcr.2003.12.003.
So EY, Ausman M, Saeki T, Ouchi T. Phosphorylation of SMC1 by ATR is required for desferrioxamine (DFO)-induced apoptosis. Cell Death Dis 2011;2(41):e128. http://dx.doi.org/10.1038/cddis.2011.9.
Saletta F, Suryo Rahmanto Y, Siafakas AR, Richardson DR. Cellular iron depletion and the mechanisms involved in the iron-dependent regulation of the growth arrest and DNA damage family of genes. J Biol Chem 2011;286(41):35396-406. http://dx.doi.org/10.1074/jbc.M111.273060.
Bonini MG, Rota C, Tomasi A, Mason RP. The oxidation of 2',7'-dichlorofluorescin to reactive oxygen species: A self-fulfilling prophesy? Free Radic Biol Med 2006;40(6):968-75. http://dx.doi.org/10.1016/j.freeradbiomed.2005.10.042.
Lawrence A, Jones CM, Wardman P, Burkitt MJ. Evidence for the role of a peroxidase compound I-type intermediate in the oxidation of glutathione, NADH, ascorbate, and dichlorofluorescin by cytochrome c/H2O2. Implications for oxidative stress during apoptosis. J Biol Chem 2003;278(32):29410-9. http://dx.doi.org/10.1074/jbc.M300054200.
Robinson KM, Janes MS, Pehar M, Monette JS, Ross MF, Hagen TM, et al. Selective fluorescent imaging of superoxide in vivo using ethidium-based probes. Proc Natl Acad Sci U S A 2006;103(41):15038-43. http://dx.doi.org/10.1073/pnas.0601945103.
Muñoz P, Humeres A, Elgueta C, Kirkwood A, Hidalgo C, Núñez MT. Iron mediates N-methyl-D-aspartate receptor-dependent stimulation of calcium-induced pathways and hippocampal synaptic plasticity. J Biol Chem 2011;286(15):13382-92. http://dx.doi.org/10.1074/jbc.M110.213785.
Li GF, Xu YJ, He YF, Du BC, Zhang P, Zhao DY, et al. Effect of hepcidin on intracellular calcium in human osteoblasts. Mol Cell Biochem 2012;366(1-2):169-74. http://dx.doi.org/10.1007/s11010-012-1294-y.
Hanahan D, Weinberg RA. Hallmarks of cancer: The next generation. Cell 2011;144(5):646-74. http://dx.doi.org/10.1016/j.cell.2011.02.013.
Roderick HL, Cook SJ. Ca2+ signalling checkpoints in cancer: Remodelling Ca2+ for cancer cell proliferation and survival. Nat Rev Cancer 2008;8(5):361-75. http://dx.doi.org/10.1038/nrc2374.
Prevarskaya N, Skryma R, Shuba Y. Calcium in tumour metastasis: New roles for known actors. Nat Rev Cancer 2011;11(8):609-18. http://dx.doi.org/10.1038/nrc3105.
Fiorio Pla A, Avanzato D, Munaron L, Ambudkar IS. Ion channels and transporters in cancer 6. Vascularizing the tumor: TRP channels as molecular targets. Am J Physiol Cell Physiol 2012;302(1):C9-15. http://dx.doi.org/10.1152/ajpcell.00280.2011.
Rizzuto R, Pinton P, Ferrari D, Chami M, Szabadkai G, Magalhães PJ, et al. Calcium and apoptosis: Facts and hypotheses. Oncogene 2003;22(53):8619-27. http://dx.doi.org/10.1038/sj.onc.1207105.
Lehen'kyi V, Shapovalov G, Skryma R, Prevarskaya N. Ion channnels and transporters in cancer 5. Ion channels in control of cancer and cell apoptosis. Am J Physiol Cell Physiol 2011;301(6):C1281-9. http://dx.doi.org/10.1152/ajpcell.00249.2011.
Ramsey IS, Delling M, Clapham DE. An introduction to TRP channels. Annu Rev Physiol 2006;68:619-47. http://dx.doi.org/10.1146/annurev.physiol.68.040204.100431.
Berridge MJ. Elementary and global aspects of calcium signalling. J Exp Biol 1997;200:315-9. http://dx.doi.org/10.1113/jphysiol.1997.sp021927.