Control of body temperature and immune function in patients undergoing open surgery for gastric cancer
DOI:
https://doi.org/10.17305/bjbms.2018.2552Keywords:
Gastric cancer, heating methods, infection, perioperative period, CD4 CD25 Treg, TGF-β, IL-10Abstract
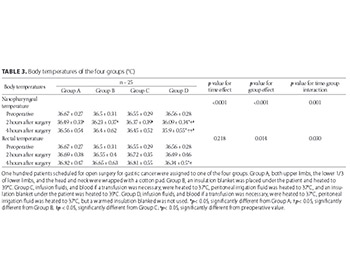
The influence of mild perioperative hypothermia on the immune function and incidence of postoperative wound infections has been suggested, but the specific mechanism is unclear. This study aimed to analyze the body temperature, immune function, and wound infection rates in patients receiving open surgery for gastric cancer. Body temperature was controlled in each patient using one of four different methods: wrapping limbs, head and neck; insulated blankets; warming infusion fluids and insulated blankets; and warming fluids without insulated blankets. One hundred patients were randomly divided into four groups of 25 patients each, and every group received a different intraoperative treatment for maintaining normal body temperature. Nasopharyngeal and rectal temperatures, transforming growth factor beta (TGF-β), interleukin 10 (IL-10) levels, and cluster of differentiation (CD)3+T and CD4+/CD25+ regulatory T cell (Treg) counts were measured before surgery and at 2 and 4 hours postoperatively. Patients were evaluated at one week after surgery for signs of infection. Intraoperative body temperature and measures of immune function varied significantly between the four groups, with the largest temperature changes observed in the group in which only the limbs were wrapped in cotton pads to control the body temperature. The lowest temperature change (i.e., close to normal temperature) and cytokine response after surgery were observed in the group in which infusion fluids and transfused blood (if needed) were heated to 37℃, peritoneal irrigation fluid was heated to 37℃, and an insulation blanket was heated to 39℃ and placed under the patient. No intergroup differences were found in the infection rates at one week after surgery. In conclusion, body temperature variation during surgery affects the immune function of patients, and maintaining body temperature close to normal results in the least variation of immune function.
Downloads
References
Balayssac D, Pereira B, Bazin JE, Le Roy B, Pezet D, Gagniere J. Warmed and humidified carbon dioxide for abdominal laparoscopic surgery: Meta-analysis of the current literature. Surg Endosc 2017;31(1):1-12. https://doi.org/10.1007/s00464-016-5335-6; https://doi.org/10.1007/s00464-016-4866-1.
Jeyadoss J, Thiruvenkatarajan V, Watts RW, Sullivan T, van Wijk RM. Intraoperative hypothermia is associated with an increased intensive care unit length-of-stay in patients undergoing elective open abdominal aortic aneurysm surgery: A retrospective cohort study. Anaesth Intensive Care 2013;41(6):759-64.
Mehta OH, Barclay KL. Perioperative hypothermia in patients undergoing major colorectal surgery. ANZ J Surg 2014;84(7-8):550-5. https://doi.org/10.1111/ans.12369.
Jing YM. Advances in the etiology and preventive measures of intra-operative hypothermia. [Article in Chinese]. Guide China Med 2013;1134:63-5.
Cui XM, Yu J, Wu J. Influence of intra-operative hypothermia on the recovery from anesthesia. [Article in Chinese]. China Health Ind 2014;11:165-6.
Kurz A, Sessler DI, Lenhardt R. Perioperative normothermia to reduce the incidence of surgical-wound infection and shorten hospitalization. Study of wound infection and temperature group. N Engl J Med 1996;334(19):1209-15. https://doi.org/10.1056/NEJM199605093341901.
Liu Y, Li L. Clinical study on the effects of temperature holding nursing care on the intra-operative stress and postoperative anesthesia recovery in patients under general anesthesia. [Article in Chinese]. Hebei Med 2014;20:1200-3.
Zhang SY, Zhu JY, Peng YZ, Sun N, Zhang RM. Nursing intervention and effects of hypothermia in postoperation to postanesthesia recovery period. [Article in Chinese]. Chinese J Nurs 2003;3:16-8.
Zhao ZM. Intra-operative application of heated fluid for infusion and irrigation in the care for hypothermia. [Article in Chinese]. Women’s Health Res 2015;20:26-6.
Wang ZN. Clinical Observation on Forced air Warming Blanket to Prevent Patients with Peri-operative Hypothermia Undergoing Radical Gastrectomy. Shanghai: Shandong University; 2014.
Beilin B, Shavit Y, Razumovsky J, Wolloch Y, Zeidel A, Bessler H. Effects of mild perioperative hypothermia on cellular immune responses. Anesthesiology 1998;89(5):1133-40. https://doi.org/10.1097/00000542-199811000-00013.
Marek-Trzonkowska N, Piekarska K, Filipowicz N, Piotrowski A, Gucwa M, Vogt K, et al. Mild hypothermia provides Treg stability. Sci Rep 2017;7:17940. https://doi.org/10.1038/s41598-017-17186-4.
Beyer M, Kochanek M, Darabi K, Popov A, Jensen M, Endl E, et al. Reduced frequencies and suppressive function of CD4+CD25hi regulatory T cells in patients with chronic lymphocytic leukemia after therapy with fludarabine. Blood 2005;106(6):2018-25. https://doi.org/10.1182/blood-2005-02-0642.
Adriani MB, Moriber N. Preoperative forced-air warming combined with intraoperative warming versus intraoperative warming alone in the prevention of hypothermia during gynecologic surgery. AANA J 2013;81(6):446-51.
Campbell G, Alderson P, Smith AF, Warttig S. Warming of intravenous and irrigation fluids for preventing inadvertent perioperative hypothermia. Cochrane Database Syst Rev 2015;(4):CD009891. https://doi.org/10.1002/14651858.CD009891.pub2.
de Bernardis RC, Siaulys MM, Vieira JE, Mathias LA. Perioperative warming with a thermal gown prevents maternal temperature loss during elective cesarean section. A randomized clinical trial. Braz J Anesthesiol 2016;66(5):451-5. https://doi.org/10.1016/j.bjane.2014.12.007.
John M, Crook D, Dasari K, Eljelani F, El-Haboby A, Harper CM. Comparison of resistive heating and forced-air warming to prevent inadvertent perioperative hypothermia. Br J Anaesth 2016;116(2):249-54. https://doi.org/10.1093/bja/aev412.
Pu Y, Cen G, Sun J, Gong J, Zhang Y, Zhang M, et al. Warming with an underbody warming system reduces intraoperative hypothermia in patients undergoing laparoscopic gastrointestinal surgery: A randomized controlled study. Int J Nurs Stud 2014;51(2):181-9. https://doi.org/10.1016/j.ijnurstu.2013.05.013.
Zeba S, Surbatović M, Marjanović M, Jevdjić J, Hajduković Z, Karkalić R, et al. Efficacy of external warming in attenuation of hypothermia in surgical patients. Vojnosanit Pregl 2016;73(6):566-71. https://doi.org/10.2298/VSP150330032Z.
Shao L, Tuerhongjiang TX, Jia FJ, Yan QY. Effects of different warming methods on interleukin in peripheral blood for patients undergoing radical gastrectomy. [Article in Chinese]. J Nurs Sci 2015;3024:42-4.
Billeter AT, Rice J, Druen D, Sklare S, Walker S, Gardner SA, et al. Warming to 39°C but not to 37°C ameliorates the effects on the monocyte response by hypothermia. Ann Surg 2016;263(3):601-7. https://doi.org/10.1097/SLA.0000000000001175.
Puttick MI, Scott-Coombes DM, Dye J, Nduka CC, Menzies-Gow NM, Mansfield AO, et al. Comparison of immunologic and physiologic effects of CO2 pneumoperitoneum at room and body temperatures. Surg Endosc 1999;13(6):572-5. https://doi.org/10.1007/s004649901043.
Schlegel A, Kron P, Graf R, Clavien PA, Dutkowski P. Hypothermic oxygenated perfusion (HOPE) downregulates the immune response in a rat model of liver transplantation. Ann Surg 2014;260(5):931-7. https://doi.org/10.1097/SLA.0000000000000941.
Jost NH, Abel S, Hutzler M, Sparwasser T, Zimmermann A, Roers A, et al. Regulatory T cells and T-cell-derived IL-10 interfere with effective anti-cytomegalovirus immune response. Immunol Cell Biol 2014;92(10):860-71. https://doi.org/10.1038/icb.2014.62.
Centers for Disease Control and Prevention (CDC). Surgical Site Infection (SSI). Available from: https://www.cdc.gov/hai/ssi/ssi.html. [Last accessed on 2018 Jan 12].
Liang MK, Goodenough CJ, Martindale RG, Roth JS, Kao LS. External validation of the ventral hernia risk score for prediction of surgical site infections. Surg Infect (Larchmt) 2015;16(1):36-40. https://doi.org/10.1089/sur.2014.115.
Ikeda T, Sessler DI, Marder D, Xiong J. Influence of thermoregulatory vasomotion and ambient temperature variation on the accuracy of core-temperature estimates by cutaneous liquid-crystal thermometers. Anesthesiology 1997;86(3):603-12. https://doi.org/10.1097/00000542-199703000-00012.
Taguchi A, Ratnaraj J, Kabon B, Sharma N, Lenhardt R, Sessler DI, et al. Effects of a circulating-water garment and forced-air warming on body heat content and core temperature. Anesthesiology. 2004;100(5):1058-64.
Lin WH, Zhang QL, Qian HJ. Effects of CO2 pneumoperitioneum on patient’s temperature and shivering during laparoscopic operation. [Article in Chinese]. Chinese J Nurs 2007;42:953-4.
Weirich TL. Hypothermia/warming protocols: Why are they not widely used in the OR? AORN J 2008;87(2):333-44. https://doi.org/10.1016/j.aorn.2007.08.021.
Good KK, Verble JA, Secrest J, Norwood BR. Postoperative hypothermia – The chilling consequences. AORN J 2006;83(5):1054-66.
https://doi.org/10.1016/S0001-2092(06)60116-6.
Rajagopalan S, Mascha E, Na J, Sessler DI. The effects of mild perioperative hypothermia on blood loss and transfusion requirement. Anesthesiology 2008;108(1):71-7. https://doi.org/10.1097/01.anes.0000296719.73450.52.
Sawamukai N, Satake A, Schmidt AM, Lamborn IT, Ojha P, Tanaka Y, et al. Cell-autonomous role of TGFβ and IL-2 receptors in CD4+ and CD8+ inducible regulatory T-cell generation during GVHD. Blood 2012;119(23):5575-83.
https://doi.org/10.1182/blood-2011-07-367987.
Wang LJ, Miao TG, Ning GX, Zhang JJ, Cai LF. The influence of TACE on T lymphocyte subsets in patients with primary liver cancer. [Article in Chinese]. J Intervent Radiol 2015;2402:165-8.
Magenau JM, Qin X, Tawara I, Rogers CE, Kitko C, Schlough M, et al. Frequency of CD4(+)CD25(hi)FOXP3(+) regulatory T cells has diagnostic and prognostic value as a biomarker for acute graft-versus-host-disease. Biol Blood Marrow Transplant 2010;16(7):907-14. https://doi.org/10.1016/j.bbmt.2010.02.026.